ES Journal of Neurology
ISSN: 2768-0606
Where Darwin Neglected to Explain the Human-Brain Encephalization: 2). The Biochemical Model Supporting the “Savanah Dry-Land Hypothesis” (SDLH)
Review article
- Vincent van Ginneken*
- Blue-Green Technologies, Heelsum, Netherlands
- *Corresponding author: Vincent van Ginneken, (PhD-1, PhD-2, MSc), Blue-Green Technologies, Heelsum, Netherlands, Email: vvanginneken@hotmail.com
- Received: March 07, 2021; Accepted: April 05, 2021; Published: April 09, 2021
- Copyright: 2021 © Vincent van Ginneken. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
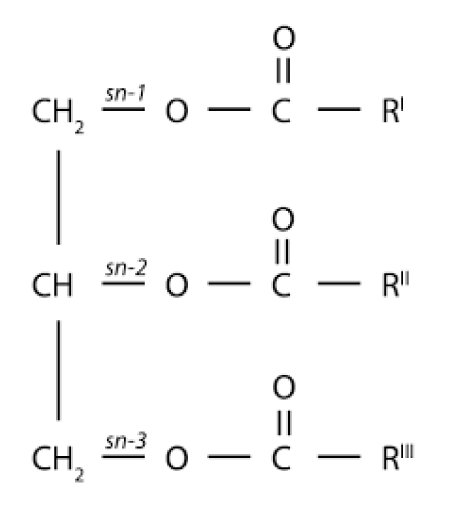
In an earlier review, we suggested the African savannah with its enormous herds of the ancestors of the African buffalo whas the 'driving force' in human encephalization delivering the substances essential to early hominids for their tremendous brain expansion from Homo erectus (average brain volume about 950 cm3 for the African lineage) towards modern Homo sapiens (≈ 1500 cm3 brain). In this review, we argue that the meat and lard of early African bovines (ancestors of the African Buffalo, Syncerus caffer) had the appropriate biochemical composition to deliver the necessary polyunsaturated fatty acids (PUFAs) such as the omega 3 fatty acids EPA (Eicosapentaenoic acid; C20: 5, ω-3) and DHA (Docosahexaenoic acid; C22: 6, ω-3), required for healthy brain growth. The most important fatty acid for the African buffalo meadow-grasses (mainly Andropogon gavanus) of which the major important Essential Fatty acid (EFA) is an inflammatory one, an ω-6, Linoleic acid (C18: 2, ω-6; LA). This will have affected the meat and lard composition of Syncerus caffer as prey animal for early hunting hominids. Therefore, it is not remarkable that fatty acids in the ω-6 series like Arachidonic acid (C20: 4, ω-6) are more common in the tissues of the African buffalo than of the ω-3 series. Remarkable is that the most important ω-3 fatty acid in this group was Docosapentaenoic acid (C22: 5, ω3; DPA). Data on meat of several African ruminants like the giraffe, gnu and several African antilopes support the general observation that all ruminants of the African savannah contain substantial amounts (about 7-12%) of Docosahexaenoic acid (C22: 5, ω3; DPA) but little (about 1-5%) Docosahexaenoic acid (C22: 6, ω-3; DHA) and Osbond acid (C22: 5, ω-6; OA) in liver and muscle tissue. It was suggested that these observations reflect a low capacity for ∆4 desaturation in these species. However, this ∆4 desaturation model has been replaced by another biochemical model whereby the biosynthesis of DHA in vertebrates is achieved by two consecutive elongations from EPA to produce Tetracosapentaenoic acid (C24: 5, ω−3; TPA) which then undergoes a ∆6 desaturation to form Tetracosahexaenoic acid (C24: 6, ω-3; THA), the latter being β- oxidized to DHA in peroxisomes. This pathway is known as the ‘Sprecher’s shunt’. Future studies must prove if a variety of biosynthetic pathways of PUFA conversions is present such as ∆3, ∆4, ∆5, ∆6, ∆8, ∆9, ∆ 12, ∆15, ∆17 desaturase enzymatic activity in the ruminant Syncerus caffer using a microbiome in the paunch dependent on the substrates consumed. Conjugated Linoleic acid (CLA) is naturally produced in the rumen in the African buffalo by bio-hydrogenation, hence it is present in milk, dairy products, and meat. In this case, the predominant isomer is the cis-9, trans-11, also known as rumenic acid. CLA and its metabolites are incorporated in neutral lipids as in adipose and mammary tissues which are rich in triacylglycerols like in human brain. At the end of this review, we propose a two-year research project to investigate the evolutionary important topic of the ‘overgrown human brain’. Palmitic acid (C16:0) has a dominant role at the sn-2 position of the glycerol molecule which explains its high absorption rate. By describing our ‘African Buffalo Savannah Hypothesis’ (ABS hypothesis) in this way to the proponents of the ‘Aquatic Phase Hypothesis’ (APH), we provide the required biochemical model for supportive action of the ‘Savannah Dry Land Hypothesis’ (SDL) hypothesis by which the encephalization of the human brain can be explained as solely dependent on molecular building materials from the African savannah.
Keywords: Homo sapiens; Human brain; Neocortex; Encephalization; Syncerus caffer; C57bl6; Encephalization; Human brain evolution; Sprecher’s shunt; Out of Africa; Systems Biology; Lipidomics§1: Introduction
Introduction
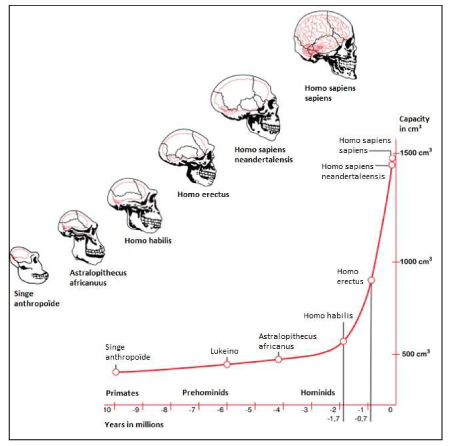
The African savannah hypothesis plays a prominent role in this review manuscript and in the formulation of our initial hypothesis related to human brain encephalization. In this respect, mitochondrial DNA studies proved extremely important supporting the proposal by [1] that a large and diverse human population has persisted in eastern Africa and that eastern Africa may be the cradle of humanity [2]. Genetic studies and fossil evidence indicate that archaic humans evolved to anatomically modern humans solely in Africa between 200,000 and 60,000 years ago [3]. In addition, they suggested that members of one branch of Homo sapiens left Africa at some point between 125,000 and 60,000 years ago, and that over time these humans replaced other populations of the genus Homo such as Neanderthals and Homo erectus [4]. The huge African savannas of thousands of square kilometers - also called ‘biomes’ - underwent another phase of change during the Pleistocene and this change, including tall grass, had its influence on the first humanoids. In this regard, human adaptation began with the first significant increase in skull volume of Homo erectus that exhibited life strategies similar to Homo sapiens about 1.9 million years ago. The prominent change was the increased relative brain size that separated Homo erectus from Australopithecines Figure (1) [5].
We will only focus on the brain growth of Homo habilis (2.4 million to 1.4 million years ago with a skull capacity of around 600 cm3 [6] to the last 75,000 years when brain growth was exponential from Homo erectus (on average about 950 cm3 for African descent, [7] to modern Homo sapiens Figure 1,2 on average about 1260 cm3 for men and 1130 cm3 for women, although there is considerable individual variation [8].
We hypothesize triacylglycerols (TGs) were the trigger for human encephalization which was the growth factor for logarithmic skull expansion Homo habilis towards Homo erectus over around 100,000 years and from Homo erectus towards Homo sapiens during the last 70,000 years.
East Africa is a region of often harsh and unsTable environments and resources are accordingly scarcer and harder to get. It is strange that the cradle for modern humanity stood here but it might due to a variety of evolutionary selection pressures in such a versatile environment selecting these evolutionary traits by an endless series of ‘breedings’ and ‘eatings’ out of which some traits developed and became what we see today in the modern archaic men of AD 2020 [9]. Species living in this latter environment would be under greater selection pressure to evolve and change if they needed to survive. The ancestors of modern humans were able through extensive use of tools and the use of gestures for communication and perhaps communication through sound or even primitive language, to exploit new skills improving their hunting techniques in the savannah, resulting in a steadily increasing amount of meat which in turn resulted in an increasing brain growth. So, an improved tool-use facility would contribute to increasing the survival chances of the species and resulting in obtaining more available meat from hunted prey animals like Syncerus caffer as was probably the case in the transition from Homo habilis to Homo erectus [9].
In this regard, it should be noted that the food sources produced by the African savannah are an extremely important factor in our model of human brain growth (encephalization). These food sources for the first hominids consisted in particular of the ancestors of the African buffalo (Syncerus caffer), the most important ruminant herbivore at the African savannah with herds of around 1,000 animals each of which an has a shoulder height between 1.0 and 1.67 m, a head and body length of between 2.1 and 3.0 m, a weight for an adult bull between 600 and 900 kg, and a life expectancy of between 18 and 29 years (reviewed: [10]. So, these herds of African buffalos, trafficking across the African savannah, can be considered as tremendous resources of “animal-meat” providing the ‘carrying capacity’ of the African savannah in terms of protein, fat and lard production required by the developing early hominids, mainly in respect to human brain growth.
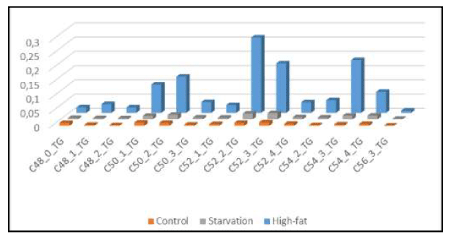
In earlier studies in a High-Fat Diet obesity induced C57bl6 mouse model fed on 24.0% bovine lard, we observed accumulation of specific Triacylglycerols (TGs) in liver (‘hepatic steatosis’) under conditions of starvation [11] but also after exposure to a High-fat diet based on bovine lard [12]. Our results depicted in Figure 3 support the hypothesis that large amounts of TGs were the 'prime movers' in brain evolution for skull expansion (encephalization) [13]. Therefore, we hypothesize that the unique lipid composition of bovine lard (large amounts of unsaturated TGs: C: 50-1; C:50-2; C:52-2; C:52-3; C54-3; C:54-4 and C56-3) might play a role in mammalian encephalization Figure 3, [14].
The specific molecular structure of bovine lard (high amounts of unsaturated C:50-1; C50-2; C:52-2; C:52-3; C54-3; C:54-4 and C56-3 Triacylglycerols [12]. We found a Pearson’s correlation coefficient of r2=0.421 for the high-fat (HF) diet and the HF brain in comparison to control- chow diet r2= -0.273 and control-brain.
To our awareness, brain expansion through nutritional intervention has never before been observed in a mouse model related to the accumulation of TGs due to a fat-based diet on bovine lard. We have extended this observation to the research field of paleoanthropology and encephalization of early hominids and via this route we came to the "Out of Africa" (OOA) hypothesis. We are aware that we are not specialists and that this area of research is completely new to us. However, our observation may be valuable; just like modern mitochondrial DNA techniques gave a major "leap forward" in the research field of paleo-anthropology, this could also be the case with the help of a Systems Biology, lipidomics-based approach using LCMS techniques in mouse models or in post mortem human brain, because our evolution is literally to our mind engraved in us human brain. To make our initial thought more structured,
African buffalo meat has - according to our hypothesis - a specific TG composition making it easy to cross the blood-brain barrier (BBB) subsequently triggering brain growth [15. We recalculated earlier determined correlation coefficients published by [12] for the food-brain correlation and found a tight correlation with the HF- diet mouse brain composition with respect to these TGs for the HF-food diet. We found a new Pearson’s correlation coefficient of r2=0.421 for the high-fat (HF) diet and the HF brain in comparison to control-chow diet r2= -0.273 and control-brain. Dietary quality has played a prominent role in theories of human evolution in general and the evolution of the human brain in particular [16]. Ideas of brain evolution centring on dietary quality until present were reviewed earlier [9] and were not confined to humans and human evolution [17,18] coined the “Extractive Foraging Hypothesis” to explain the relationship in primates. They argued that a relatively large brain correlates with omnivorous feeding in primates, which requires relatively complicated strategies for extracting high quality foodstuffs. The importance of a high-quality diet and of eating meat in particular, has been a common theme [19]. In an earlier publication, we suggested that tremendous herds of the African Buffalo (Syncerus caffer) (Figure 4) provided the meat and lard for transformation of the brains of early hominids towards that of modern men Homo sapiens at the African savannah [9].
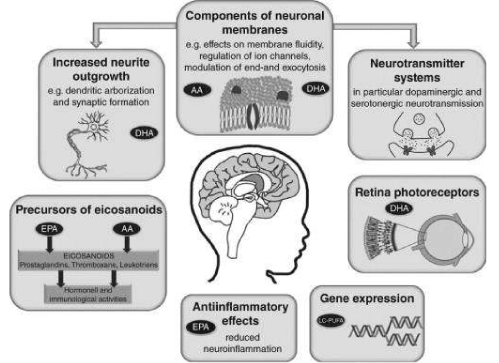
The biological activity of omega-3 fatty acids (ω-3 FAs) has already been extensively studied for several decades, and its role in brain function and mental health is of the utmost importance from a qualitative point of view [20,21]. For its growth and development, the human brain undeniably needs large amounts of the polyunsaturated fatty acid (PUFA) "fishy oil" Eicosapentaenoic acid (C20: 5, omega-3; EPA), in addition to the other "fishy oil" Docosahexaenoic acid (C22: 6, omega-3; DHA).
Because EPA and DHA can be obtained from fish and mussels which re-extract these two PUFA compounds from microalgae - in addition to the direct consumption of seaweeds by hominids [22] - a hypothesis evolved that humanity during its evolution and development of the brain (encephalization) obtained the required EPA and DHA via an aquatic sea phase. This is also called the Aquatic Phase Hypothesis (APH) [23], which directly opposes that of the research school of the (African) Savannah Dryland Hypothesis (SDLH) [24]. The latter suggests that the African savannah provided sufficient nutrients (such as the above two PUFAs, EPA and DHA, in addition to the essential micro-element iodine) for human brain growth of Homo erectus in line with modern humans Homo sapiens over a period of 200,000 years [9].
So, presently there are still in evolutionary sciences two contradictory theories: the “Aquatic Phase Hypothesis” (APH) [23,24] and the “Savannah Dry Land Hypothesis” (SDLH) [25]. In "On the Origin of Species" [26] as well as "In the Descent of Man" [27], Charles Darwin - himself a supporter of the (African) SDLH - was unable to provide the biochemical evolutionary model that could explain the growth spurt of the human brain during these 200,000 years due to a lack of advanced biochemical analytical laboratory equipment.
So, in summary, there exist presently two mainstream hypotheses related to human brain encephalization. The ‘Savannah Dry Land Hypothesis’ (SDLH) assumes that the African savannah would have supplied all nutritional elements and other chemical compounds to explain the uniqueness of the human brain with its overgrown neocortex [25,28]. On the other side of this scientific paradigm [29]. we have the ≈60-year-old 'Aquatic Phase Hypothesis' (APH) or Hardy / Morgan hypothesis [30] which states that our ancestors were evolutionarily adapted to a water phase [24,31,32]. The followers of the APH evolution model request the SDL supporters for a biochemical explanatory model for large human brains [32]. We will propose in this review such a biochemical model of human brain encephalization based on literature data and in a third review to be published following a Systems Biology lipidomics approach at our final data. We used material of sixteen brain donors (eight Control and eight type 2 diabetes) of the “Netherlands Brain Bank” for human brain (neocortex) and human blood plasma and a supportive C57bl6 mouse group model (Control-. High-fat diet and 24 h starvation group) for whole brain homogenate and blood plasma.
Thus, when explaining the evolutionary trait "walking on two legs" – which presumably evolved as evolutionary trait induced by high savannah grass [9]- in combination with improved tools for hunting which resulted in more meat, this model explains all the aforementioned anatomical and physiological characteristics [29]. It is most obvious to follow a scenario of evolutionary selection drivers - that logically explain most physiological and anatomical features of modern Homo sapiens - which are based on this savannah hunting model. These are in chronological order: a). large brains evolved because of complex social organization due to hunting for the tremendous herds of the (ancestors) of the African buffalo (Syncerus caffer) see Figure 5 [9]; b). required higher intelligence; c). the subcutaneous fat layer developed to serve as an energy reserve for the developing brain; d). articulated speech evolved because there was social pressure for extensive communication; e). the larynx decreased because this was required by articulated speech [9]; f). bipedalism [33] as evolutionary trait increased velocity during hunt and made the use of tools and weapons easier; g). and a naked body evolved to prevent overheating during hunting [29,32], almost convulsively adhere firmly to the APH hypothesis while they state: “There is little or any evidence of these in the fossil record and much of what has been claimed can be explained in other ways”.
In order to complete this overview, paleoanthropological indications for archaic humans in an APH model may be mentioned: a). they have been found in South Africa [34]; b). for Neanderthals [35]; c). early human-like food has included various land and aquatic animals since late quaternary and at least intermittent since
1.9 Ma [36]; d). water and sea behavioral changes (e.g., boats, tools for fishing) have been observed probably since the last interglacial [34]. In addition, paleoanthropological indications of ‘marine habitats’ refers to the open ocean and mention can be made of the occurrence of pelagic fish in Melanesia at 43Ka [38] and the likely occupation of islands such as Flores at the start of the Middle towards Late Pleistocene [39].
In my opinion, however, after the Australepthicus (Figure 1) about 4 to 5 million years ago [40], the evolution from the first hominins & hominids to modern archaic man took place on the African savannah. Personally, I am of the opinion that the APH hypothesis may apply only for the period after the Toba volcano disaster about 75,000 years ago [9,41], when modern people spread around the world and occupied the different continents [9]. I will substantiate this by first presenting the biochemical model for the growth spurt of our human brain based on the SDLH that the APH supporters asked. We strongly believe that after the Toba volcano eruption some 75,000 years ago, "the survivors" traveled around the world, mostly along a coastline [34]. But the fact remains that the increase of the brain size of modern man took place on the African savannah. So, the first archaic human with its '1500cm3' skull volume had its cradle on the African savannah which is firstly based on fossil excavations [2], secondly on genomic DNA studies [42], and thirdly on our ecological model in which the ancestors of the African buffalo (Syncerus caffer) produced those amounts of meat and lard with the right biochemical composition (this review) on the African savannah which the early hominids needed for proper brain development. So, we will describe an SDL-hypothesis which explains how the human brain could develop without the need of an aquatic phase. In this review, we will provide a biochemical model that supports the SDLH-following a System Biology [43], lipidomics based approach [44,45].
In addition, presently, there is an important “unspoken” dichotomous approach in evolutionary sciences in which a lipid fraction plays a major role in human brain encephalization: the omega-3 PUFAs (Eicosapentaenoic acid: EPA & Docosahexaenoic acid: DHA) and other VLCFA interconversions or the Triacylglycerol fraction (TGs). Earlier, we described the observation in a C57bl6 High-Fat diet induced obese mouse model fed on bovine lard with large amounts of unsaturated TGs C50:1; C:50-2; C:52-2; C:54-3; C:54-4 and C:56-3 which might have played a role in mammalian encephalization [12]. Our observations support this new insight suggesting these TGs can pass the Blood Brain Barrier (BBB) and fuel the modern human brain. Consequently, TGs accumulation or “brain steatosis” is an important observation [12]. Earlier, [46] made a similar observation that fatty acids as long as 36 carbon atoms are present in significant amount in the brain of vertebrates, including mammals and humans. In addition, to our awareness only one nutritional intervention study which is comparable to our rodent study was performed with feline [47]. In this study, using deuterium labelled FA tracers, domestic cats were given a diet with meat for six months which also resulted in VLCFA accumulation in the brain [47].
So, our C57bl6 mouse model, with its brief reproduction period, is a new 'tool' which gives the evolutionary biologist unprecedented and unlimited possibilities to test the various physiological, biochemical and genetic processes of evolution in a mammal in the laboratory.
The Cape buffalo weighs anywhere from 400 to 800 kg (880–1760 lbs), whereas the African forest buffalos are much lighter, weighing between 250 and 320 kg (550–705 lbs). [10,48]. In Kainji Lake National Park, Nigeria a total Syncerus caffer biomass of 54.6 ±10.9 kg km-2 was recorded [49].
So probably, during the course of human evolution, these TGs were the trigger for human encephalization and these molecules were probably the growth factor for logarithmic skull expansion from Homo erectus towards Homo sapiens during course of evolution, over a time- period of around 200,000 years [50]; Figure 2). This can be visualized with current data on the evolution of hominids, starting with Australopithecus - a hominid from which modern man are probably descended [51]. Australopiths lived from 3.85-2.95 million years ago with an overall skull capacity somewhere close to that of the existing chimpanzee, i.e. about 300 - 500 cm3 [51,52]. Given that the modern human brain measures around 1,352 cm3 on average, this represents a significant amount of developed brain mass. It is estimated that Australopiths have a total number of neurons of about 30-35 billion [6].
Moving along the human ancestral timeline (‘Encephalization-TOL’; Figure 1 & 2), the brain size continues to increase steadily (see Hominidae) when moving from primates into the Homo era. Homo habilis for example -characterized by hand bones that suggested to have an ability to manipulate objects and tools- who lived 2.4 million to 1.4 million years ago had a skull capacity of around 600 cm3. This Homo habilis is claimed to have been the first Homo species -based on a large number of characteristics- and to have had an estimated number of 40 billion neurons [6]. Brain size in Homo erectus averages about 950 cm3, while in a series of Middle Pleistocene crania from Africa and Europe, volume amounted to about 1230 cm3 [7]. Homo heidelbergensis living somewhat closer to the present time (about 700,000 to 200,000 years ago) had a skull capacity of about 1290 cm3 and about 76 billion neurons [6]. Homo neaderthalensis, which lived 400,000 to 40,000 years ago, had a skull capacity comparable to that of modern humans at around 1500-1600 cm3 on average, with some specimens of Neanderthal with an even larger skull capacity. It is estimated that Neanderthals possessed around 85 billion neurons [6]. The increase in brain size supplemented by Neanderthals, was possibly due to their larger visual systems [53].
The adult human brain weighs on average about 1.2 - 1.4 kg, or about 2% of the total body weight [54], with a volume of approximately 1260 cm3 in men and 1130 cm3 in women, although there is considerable individual variation [8]. For the sake of convenience, we refer to the modern brain of modern Homo sapiens sapiens as the ‘≈1500 cm3’ brain, which presents a landmark in evolutionary history.
In this review, based on the SDLH, we will provide the evolutionary biochemical model requested by the APH supporters to explain the growth spurt of the human brain on the African savannah.
Evolutionary Aquatic Phase Model versus Savannah (“dry-land”) hypothesis.Modern humans have evolved with a staple source of preformed Docosahexaenoic acid (C22:6, ω-3; DHA) in the diet. To date, an important turning point in human evolution is believed to be the discovery of high-quality, easily digestible nutrients from coastal seafood and inland freshwater resources [21]. The consumption of this seafood and food from aquatic resources for several generations, by hominids first, may have coincided with the rapid expansion of gray matter in the cerebral cortex which characterizes the modern human brain. This is called the "Aquatic Phase Theory" (APH), which is currently largely disputed [29]. The DHA molecule has unique structural properties that appear to provide optimal conditions for a wide range of cell membrane functions. This has particular implications for gray matter, which is a membrane-rich tissue. The rudimentary source of DHA are marine algae [21]. Therefore, it is concentrated in fish and marine oils. Unlike the photosynthetic cells in algae and higher plants, mammalian cells lack the specific enzymes required for the de novo synthesis of α-Linolenic acid (C18:3, ω-3; ALA), the precursor for all omega-3 fatty acid syntheses.
The fossil and mitochondrial DNA evidence available thus far supports an African origin for modern humans [2,56]. Several of the earliest examples of anatomically modern human remains come from the Middle Stone Age (MSA) sites of South- Africa. Bone-tools probably from human origin are reported from the MSA levels at “Blombos” and at “Klasies River Mouth”, but similar bone-tools in Europe and the Near-East do not appear until later. Sediments bearing bone-harpoon-like points at the “Katanda” sites date to circa 90 000 years ago, suggesting that African MSA early humans not only worked with bone-tools, but also exploited fish using these hunter-tools [37]. So, in conjunction with the ‘Aquatic Phase Hypothesis’ (APH) “Shellfish-gathering” is from an archaeological perspective an early indication of “modern human behaviour”. Based on the Life-style patterns of early Homo sapiens described earlier in this review – which for rational reasoning or just opportunistic reasoning- this would have included a sea-based nutritional life-style pattern in daily existence for over an estimated period of ≈ 100,000 years. A rather limited evolutionary “time-frame” of only 0.05% in the assumed total existence time of Homo sapiens.
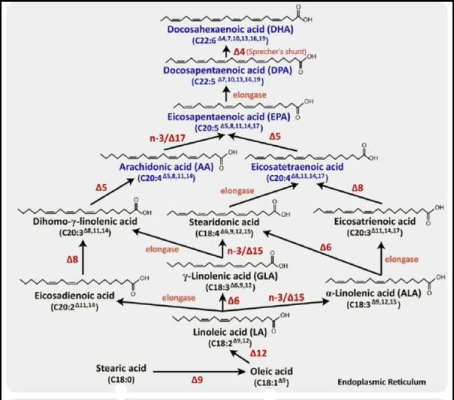
Proponents of the evolutionary ‘Aquatic Phase Model’ argue that the endogenous synthesis of Docosahexaenoic acid (C22:6, ω-3; DHA) from α-Linolenic acid (C18:3, ω-3; ALA), in humans is much lower and more limited than previously assumed by competitive enzymatic systems. Rate limiting steps in enzymatic conversions due to ‘retarded’ enzymatic reactions are the ∆6 desaturase steps for both Essential Fatty acids (EFAs), Linoleic acid (C18:2,ω-6, LA) and α-Linolenic acid (C18:3, ω-3; ALA) and the ∆5 desaturase step from Dihomo-ℽ- Linolenic acid (C20:3, ω-6; DGLA) towards Arachidonic acid (C20:4, ω-6; ARA) (Figure 6).
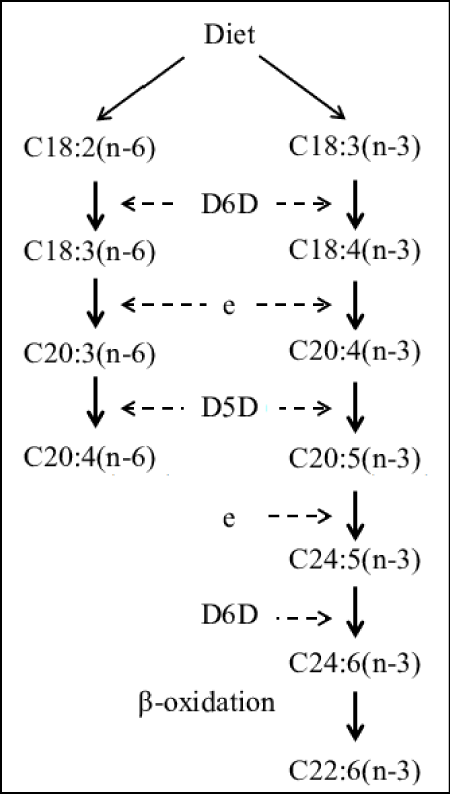
Enzym activities can be calculated based on product-to-precursor ratios of individually measured fatty acids. Rate limiting steps in enzymatic conversions due to ‘retarded’ enzymatic reactions are the ∆6 desaturase steps (D6D) for both Essential Fatty acids (EFAs), Linoleic acid (C18:2,ω-6, LA) and α-Linolenic acid (C18:3, ω-3; ALA) and the ∆5 desaturase step (D5D) from Dihomo-ℽ- Linolenic acid (C20:3, ω-6; DGLA) towards Arachidonic acid (C20:4, ω-6; ARA) and finally the ‘Sprecher’s shunt’ (Figures 8 & 24) -partly in the peroxisome- from Eicosapentaenoic acid (C20:5, ω-3; EPA) towards Docosahexaenoic acid (C22:6, ω-3; DHA).
Here, we will argue that our ecological ‘Out-of-Africa Buffalo-Savannah’ hypothesis vigorously describing the many enzymatic metabolic PUFA interconversions, provides strong evidence that the meat and lard of the herds of the African buffalo hunted by early man at the mainly Pleistocene African savannah, had such a specific biochemical composition enabling it to be the probable evolutionary driver for encephalization of the early hominids. In order to prove this hypothesis, we will provide the reader of this review with a summary of scientific literature covering all aspects related to nutrition, physiology, biochemistry, and (bio) medicine of the human brain of modern Homo sapiens. In addition, we will also
describe the physiology of the major prey animals notably the large herbivores of the African savannah Syncerus caffer as well as the interconversion of PUFAs and FAs in the rumen (paunch) because our evolutionary model is based on a blending of these research areas.
From a biomedical point of view, starting with the ω-3 PUFA α-Linolenic acid (C18:3, ω-3; ALA), ω-3 fatty acids are required for normal health, especially for the brain whereas the ‘inflammatory’ ω-6 Linolenic acid (C18:2, ω-6; LA) has important functions in cholesterol metabolism and as specific skin lipid [57].
If we follow the ∆9 elongase pathway starting from α-Linolenic acid (C18:3, ω-3; ALA) towards Eicosatrienoic acid (C20:3, ω-3; ETrA), we find this compound to be an eicosanoid precursor. The eicosanoids derived from these fatty acids have a variety of effects on the body. For example, they play a role in inflammation, fever promotion, blood pressure regulation, and blood clotting. They also influence the immune response and certain respiratory and reproductive processes [58]. This is the mild anti- inflammatory route evolving via the omega-3 pathway. The strong anti-inflammatory route evolving via the omega-6 route proceeds as follows: Arachidonic acid (C20:4, ω-6; ARA) and Docosahexaenoic acid (C22:6, ω-3; DHA) are formed from Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA), respectively, in the liver by a series of alternating desaturation (addition of a double bond) and elongation (addition of a 2-carbon unit) reactions. Preformed Arachidonic acid (C20:4, ω-6; ARA) and Docosahexaenoic acid (C22:6, ω-3; DHA) are present in the diet in meat, fish, and eggs but not in fruits, vegeTables, nuts, grains, or their products [57].
Dairy products are also exceedingly low in DHA and Arachidonic acid and in their precursor’s Linoleic acid and α-Linolenic acid so that these PUFAs need to come from other dietary sources and some by way of endogenous synthesis in the body itself [59]. This is important in case of brain development in conjunction with adjacent blood vessels. In addition, the EFAs which are assimilated into phospholipids are particularly important in the overall structure and function of both omega-6 and omega-3 fatty acids, as phospholipids maintain both the structural integrity and the critical functioning of cellular membranes throughout the body [60].
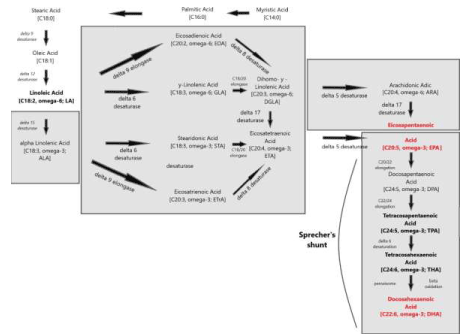
This pathway -replacing an earlier stipulated ∆4 desaturase – is called ‘Sprecher’s shunt’ [61-66].
Figure 8 presents an overview of the many optional LCPUFA interconversions and the corresponding enzymatic pathways of very recently confirmed ‘landmark’ discoveries like the Sprecher’s shunt as well as enzymatic pathways which still must be demonstrated. In the next sections, we will discuss these issues from the viewpoint of how the essential fatty acids (EFAs) Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA) are provided by Syncerus caffer at the African savannah, providing direct supportive evidence for the “African Buffalo Savannah Hypothesis” (ABS-hypothesis) [9].
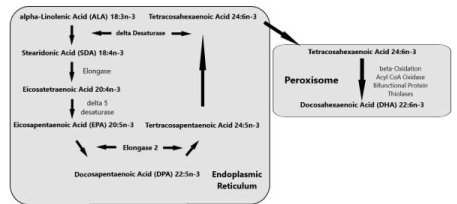
DHA in large mammals at the savannahBoth Arachidonic acid (C20: 4, ω-6; ARA) and Docosapentaenoic acid (C22: 5, ω-3; DPA) are extremely important for the developing blood vessels and human brain in early hominids. The precursor of Docosapentaenoic acid (C22: 5, ω-3; DPA), Eicosapentaenoic acid (C20:5, ω-3; EPA) was the most important ω-3 metabolite in the savannah mammals [20]. Despite its abundance, neither EPA nor the corresponding Docosapentaenoic acid (C22: 5, ω-6; DPA) was used in the photoreceptor related processes nor the synapse. Why do we emphasize the importance of organism size and growth rate in our model? The reason is that the biosynthesis of Arachidonic acid (C20: 4, 6-6; ARA) and Docosahexaenoic acid (C22: 6, ω-3; DHA) initially is relatively slow due to the rate limiting ∆5 desaturase activity for Arachidonic acid (C20: 4, 6-6; ARA) and ∆4 desaturase/ (Sprecher’s shunt) for Docosahexaenoic acid (C22: 6, ω-3; DHA). The assumed ∆4 desaturase/ (Sprecher’s shunt) enzymatic activities involved in the conversion of Docosapentaenoic acid (C22:5, ω-3; DPA) into Docosahexaenoic acid (C22:6, ω-3; DHA) may not be able to keep up with the growth of animals. It is now considered more likely that DHA is biosynthesized via a C24 intermediate, Tetracosapentaenoic acid (C24: 5 n-3; TPA), followed by β-oxidation in peroxisomes [61]. Rats and mice desaturate and extend the chain of parent essential fatty acids (EFAs) to produce larger amounts of Arachidonic acid (C20: 4, ω-6; ARA) and Docosahexaenoic acid (C22: 6, ω-3; DHA) than their predecessors [20]. With increasing size, the impact of growth rate results in a progressive decrease of Arachidonic acid (C20: 4, 6-6; ARA) and Docosahexaenoic acid (C22: 6, ω-3; DHA) while the precursors Linoleic acid (C18: 2, ω-6). ; LA) and α-Linolenic acid (C18: 3, ω-3; ALA) become more dominant in liver lipids [67]. Instead of DHA, its precursor Docosapentaenoic acid (C22: 5, ω-6; DPA) is now the major metabolite of α- Linolenic acid, such as e.g. in Syncerus caffer [20]. So, the faster an animal grows, the greater the limitation of the biosynthesis of Arachidonic acid (C20: 4, ω-6; ARA) and Docosahexaenoic acid (C22: 6, ω-3; DHA). The large African savannah mammals
- such as the ruminant African buffalo Syncerus caffer – all shared the same fate: Docosahexaenoic acid (C22: 6, ω-3; DHA) and brain capacity decreased as body size growth accelerated mainly because ‘desaturation’ and ‘elongation’ in these large mammals came to an end with regard to enzymatic conversions for the PUFA Docosapentaenoic acid (C22: 5, ω-3; DPA) so that little Docosahexaenoic acid (C22: 6, ω-3; DHA) is still synthesized [15]. What little Docosahexaenoic acid (C22: 6, ω-3; DHA) is synthesized is used in the brain and in the photoreceptor. While Docosapentaenoic acid (C22: 5, ω-3; DPA) is abundantly present in meat (muscles; see Table 1 next paragraph) of the large herbivorous mammals of the African savannah [20], this precursor of Docosahexaenoic acid (C22: 6, ω-3; DHA) is not found in the brain and photoreceptor of e.g. the African buffalo (Syncerus caffer). So, in large mammals at the savannah (e.g. Syncerus caffer), brain size was sacrificed, not brain DHA-concentration [67]. In contrast, early hominids -also inhabitants of the African Savannah- followed a different evolutionary pathway and invested in large brains [9] instead of body size as the other mammals at the savannah. In this respect, it must be remarked that all large mammals of the savannah were in principle prey animals for hunting by early hominids like the ruminant Syncerus caffer, the monogastric Zebra and the ruminant Giraffe [68]. Yet, the largest amount of biomass at the African savannah at present -and probably also in the Pleistocene- is formed by the tremendous herds of ancestors of the African buffalo (Syncerus caffer) with an estimated biomass of around of 54.6 ±10.9 kg km -2 recorded in Kainji Lake National Park, Nigeria [49].
Grazing of the ruminant Buffalo Syncerus caffer at the African savannah
Syncerus caffer grazes during the wet season but increases its territorial area in the dry season where it is also found in forest habitats [10,48,49,69]. It has access to seed material, both from the grasses and from the larger vegetation. It also changes its feeding pattern depending on the season: dry or wet. The problem with such ecological issues related to the preferred food of Syncerus caffer is that a variety of factors play a role: a) the African savannah contains 10,000 species of grass; b). Syncerus caffer has presently been studied in several regions like
e.g. the Kaainji Lake National Park Nigeria [49] and the Satara in the Central Kruger, National Park, South Africa [69]; c). depending on the dry or wet season the herbivorous menu of Syncerus caffer can change with respect to selected grasses. By chance, it has been determined that Andropogon gayanus is the most preferred grass species of Syncerus caffer at the African savannah at Kainji Lake National Park, Nigeria [10]. The preference for this grass species is due to the fact that it contains high percentages of crude protein and fat (7,60% and 5,11%, respectively) when compared to other grass species utilized by the Syncerus caffer population [10]. Next, the PUFA content of this important African savannah grass Andropogon gayanus determined by gas chromatography showed that its TGs are mainly composed of P2O, P2L, PO2, POL, O3, PL2, O2L, L2O, and L3 with P=Palmitic acid (C16:0), Oleic Acid (C18:1) and L=Linoleic acid (C18:2, ω-6; LA) [70]. So, two important conclusions can be drawn First, all combinations of these fatty acids linked to the glycerol backbone molecule can be found. Secondly, the major important EFA is an inflammatory one, an ω-6, Linoleic acid (C18:2, ω-6; LA). This will have its impact on the meat and lard composition of Syncerus caffer, the prey animal for early hunting hominids. In free-living ruminants, the major components of muscle tissue appear to be phospholipids and only small amounts of triacylglycerol. The researchers suggested that it might be useful to revise the current conception of ‘animal fat’, The fat in the muscle of the free-living animal seems to be predominantly structural in nature [71]. The infiltration of TGs in the domestic muscle obscures this characteristic. [72] major conclusion was that the relatively low proportion of polyenoic acids in meat from domestic animals might therefore be attribuTable to infiltration of the muscle by adipose tissue [72].
In addition, another extensive study of the preferred foraging grasses of Syncerus caffer performed at the Satara in the Central Kruger, National Park, South Africa, mentions the following grass species: Panicum maximum, Panicum coloratum, Urochloa mosambicensis, Heteropogon contortus, Cenchrus ciliaris; with major a preference determined for the Panicum grass species [69]. Presently LCMS or GCMS measurements of the PUFA content of ‘Panicum grass’ are lacking. So far, the in this §3 described issues this are an ecological reflection of our biochemical ABS-hypothesis in order to explain the encephalization process of the human brain.
In Figure 5, an impression is given of running animals of the African buffalo (Syncerus caffer) at the African savannah. This is a tremendous amount of ‘biomass’ considering that a herd consists of around 1,000 animals, each with a weight of approximately 800 kg. The estimated biomass of these herds of the most abundant herbivore at the African savannah determined in Kainji Lake National Park, Nigeria, amounted to a total of 54.6 ±10.9 kg km -2 [49].
It is remarkable that PUFAs of the ω-6 series are more common in the tissues of both African buffalo Syncerus caffer (Table 2; see further) and domestic cow Bos taurus (Table 1) than those of the ω-3 series. Because the C18 Linoleic acid (C18:2, ω-6; LA)) and C20 Arachidonic acid (C20:4, ω-6; ARA) PUFAs were mainly from the ω-6 series, one would expect that the C22 PUFAs (DPA & DHA) would also have the same origin. This is not the case because the most important fatty acid in this group is Docosapentaenoic acid (C22: 5, ω3; DPA). Only small amounts of Tetracosahexaenoic acid (C22: 4, ω-3; THA) and Osbond acid (C22: 5, ω-6; OA) were clearly present. In addition, also only small amounts of Docosahexanenoic acid (C22: 4, ω-3; DHA) and Docosatetraenoic acid (C22: 4, ω-6; DTA) could be detected which were considered as characteristic products of the rumen microbiome [73]. Note that these compounds are also found in the gray- and white- matter of the neocortex of the human brain [74]. No Stearidonic acid (C18: 4, ω3; STA) was detecTable indicating no activity of ∆6 desaturase, but small amounts of Eicosatetraenoic acid (C20: 4, ω3; ETA) were detected and in addition, larger amounts of ω-3 PUFA Eicosapentaenoic acid (C20: 5, ω-3; EPA) which is of major importance for the for the human brain and yet again larger quantities of the precursor and buffer PUFA Docosapentaenoic acid (C22: 5, ω3 Acid; DPA) (see Figure 8 and further Figure 22; [75] linking in this pathway EPA and the so important Docosahexaenoic acid (C22:6, ω-3; DHA). This pattern was consistent for the African savannah ruminants mentioned in Table 1 including the African buffalo (Table 2). The consistency of the polyenoic pattern between the seven different ruminant species studied here (Table 1) is remarkable.
Table 1: Concentrations of PUFAs determined by GCMS in the meat of African ruminants. Source modified: (20. Crawford et al 1969); 1. Giraffe = Giraffe camelopardalis; 2. Taurotragus oryx = eland antelope; 3. Connochaetes taurinus = Brindled gnu; 4. Kobus defassa = large antelope; 5. Acephalus buselaphus = Kongoni African antelope; 6. Damaliscus korrigum = Senegal hartebeest (African antelope); 7. Bos taurus = domestic cow. Expressed in: percentage composition of extracted fatty acids.
|
|
1). Giraffe |
2). T.oryx |
3). C.taurinus |
4). K.defassa |
5). A.buselaphus |
6).D. korrigum |
7).B. taurus |
|
Linoleic acid (C18:2, ω-6; LA) |
27 |
23 |
18 |
21 |
21 |
24 |
7.6 |
|
α-Linolenic acid (C18:3, ω-3; ALA) |
2.4 |
4.0 |
3.2 |
4.0 |
6.0 |
5.0 |
2.1 |
|
Eicosadienoic acid (C20:2, ω-6; EDA) |
0.9 |
0.2 |
0.1 |
0.1 |
0.09 |
0.1 |
0.5 |
|
Eicosatrienoic acid (C20:3, ω-3; ETrA) |
0.1 |
0.03 |
- |
- |
- |
0.05 |
- |
|
Dihomo-ℽ-Linolenic acid (C20:3, ω6; DGLA) |
0.8 |
0.1 |
0.2 |
0.1 |
0.09 |
0.1 |
0.7 |
|
Arachidonic acid (C20:4, ω-6; ARA) |
7.4 |
6.0 |
5.0 |
6.6 |
4.0 |
7.2 |
3.2 |
|
Eicosatetraenoic acid (C20:4, ω-3; ETA) |
0.4 |
0.6 |
0.09 |
0.1 |
Trace |
0.1 |
0.03 |
|
Eicosapentaenoic acid (C20:5, ω-3; EPA) |
2.1 |
1.0 |
1.05 |
1.2 |
0.9 |
1.2 |
0.9 |
|
Docosatetraenoic acid (C22:4, ω-6; DA) |
0.06 |
Trace |
Trace |
0.2 |
0.08 |
0.03 |
- |
|
Tetracosahexaenoic acid (C22:4, ω-3; THA) |
Trace |
Trace |
0.07 |
- |
0.09 |
- |
- |
|
Osbond acid (C22:5, ω-6; OA) |
0.08 |
Trace |
0.1 |
- |
- |
- |
- |
|
Docosapentaenoic acid (C22:5, ω-3; DPA) |
4.1 |
3.2 |
2.6 |
1.2 |
3.0 |
2.7 |
0.6 |
|
Docosahexaenoic acid (C22:6, ω-3; DHA) |
0.4 |
0.7 |
0.5 |
0.1 |
0.1 |
0.3 |
0.1 |
|
Total ω-6 acids |
36 |
29 |
23 |
28 |
25 |
31 |
12 |
|
Total ω-3 acids |
9 |
9 |
7 |
7 |
10 |
9 |
4 |
Since the African buffalo is a real ruminant, it is unlikely that it has access to PUFAs higher up in the metabolic pathways than Linoleic acid (C18: 2, ω-6; LA) and α-Linolenic acid (C18: 3, ω-3; ALA) in its food. The source of PUFAs with a chain length of 20 carbons or more is most likely due to the metabolism of animals. Of the EFA C18 fatty acids, Linoleic acid (C18: 2, ω-6; LA) is the most common in muscle tissue. We suggest that the source of Linoleic acid (C18: 2, ω-6; LA) is encapsulated seed material because it is rich in oil and provides a surface phase which circumvents the hydrogenating influence of the rumen. The African buffalo Syncerus caffer grazes during the wet season, but increases its surfing behavior during the dry season where it is also found in forest habitats. It has access to seed material from both the grass and the larger vegetation. The most important fatty acid of grass meadow is the α-Linolenic acid (C18: 3, ω-3; ALA) while seed material is usually a rich source of Linoleic acid (C18: 2, ω-6; LA). It is noteworthy that PUFAs of the ω-6 series are more common in the tissues of giraffe, gnu and several African antelopes than those of the ω- 3 series [20]; Table 1). In this regard, it is worth mentioning that whereas Linoleic acid (C18: 2, ω-6; LA) is predominantly included in four double bond extension products, it is α-Linolenic acid (C18: 3, ω-3; ALA) which is processed in products with five double bonds. Such a consistent finding, regardless of the ruminant species studied so far (Table 1), suggests that the PUFAs of both ranges may be important for structural purposes. This will have its impact on the biochemical composition of the meat of these African ruminants including Syncerus caffer. Now we are in a position to present an almost complete biochemical model as requested by the proponents of the “Aquatic Phase Hypothesis” (APH) research school to prove the “Savannah Dry Land Hypothesis” SDLH. Yet, it still requires a large trajectory from the biochemical meat composition of African ruminant prey animals - mainly the African buffalo Syncerus caffer- towards human brain encephalization.
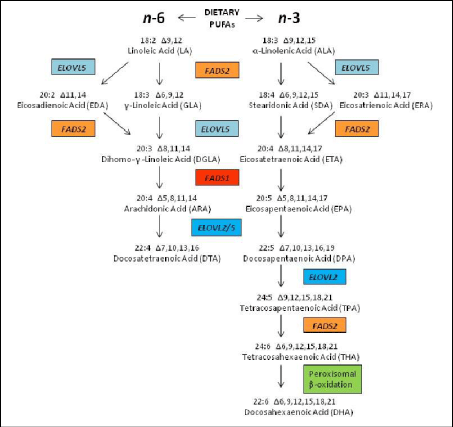
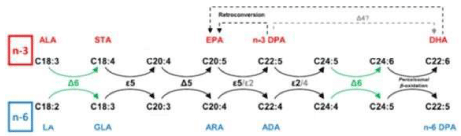
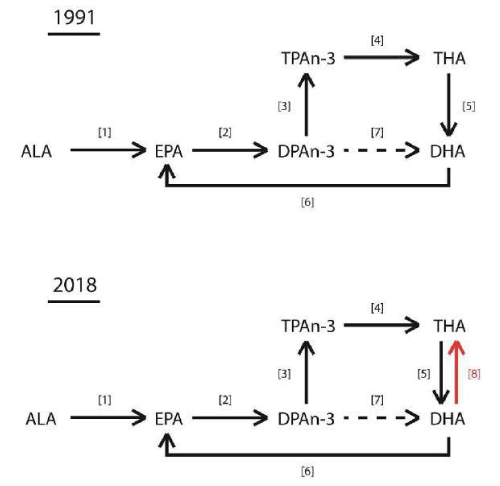
‘Sprecher’s shunt’ in detail with its encoding genesThe earlier mentioned ∆4 desaturation model for the coversion of Docosapentaenoic acid (C22:5, ω-3; DPA) into Docosahexaenoic acid (C22:6, ω-3; DHA) has been replaced by another biochemical model with a role for the peroxisomes. Since the studies of Sprecher and co-workers in rats, it has been generally accepted that the biosynthesis of DHA in vertebrates is achieved by two consecutive elongations from EPA to produce Tetracosapentaenoic acid (C24:5, ω−3; TPA), which then undergoes a ∆6 desaturation to form Tetracosahexaenoic acid (C24:6, ω-3; THA), the latter being β-oxidized to DHA in peroxisomes. This pathway mentioned earlier in this review article is known as the ‘Sprecher’s shunt’ (Figure 7; [61-66].
The pathway of which this ‘Sprecher’s shunt’ is part begins with the desaturation of α- Linolenic acid (C18:3, ω-3; ALA) forming Stearidonic acid (C18:4, ω-3; STA) by ∆6 desaturase (encoded by FADS2-genes) which is a rate limiting step. This step is followed by elongation (ELOVL1-gene) to Eicosatetraenoic acid (20:4n-3; ETA). Desaturation by ∆5 desaturase (FADS1-gene) next produces Eicosapentaenoic acid (C20:5, ω-3; EPA) which is then elongated by elongase-2 (ELOVL2-gene), first to Docosapentaenoc Acid (C22:5, ω-3; DPA) and then to Tetracosapentaenoic acid (C24:5, ω-3, TPA). Tetracosapentaenoic acid then undergoes a second ∆6 desaturation to produce Tetracosahexaenoic acid (C24:6, ω-3; THA). These initial steps occur in the endoplasmic reticulum, the final stage of DHA synthesis, however takes place in the peroxisome following translocation. In the peroxisome, Tetracosahexaenoic acid (C24:6, ω-3; THA) is shortened to DHA (22:6, n-3) by a single round of β-oxidation through the actions of acyl-coenzyme-A oxidase (ACOX1gene), D- bifunctional enzyme (HSD1784-gene) and then peroxisomal thiolases (Figure 8; [62].
During the dry season, the African buffalo is also foraging in the forest (Figure 9) and feeds undoubtedly on plant leaves. The very recent study of [76] demonstrates several interesting enzymatic conversions of PUFAs involving ω-3 desaturases (ω3Ds). Undeniably, their products will have an impact on the biochemical composition of the meat of Syncerus caffer. Although the ABS-hypothesis remains our initial major model in order to explain human brain encephalization, we will describe for the sake of completeness also this topic because plant leaves are a resource of nutrition as well for the African buffalo during the dry season when the savannah produces less grass for these tremendous herds of animals. It must be remarked that higher plants are devoid of C:20 and C:22 LC-PUFA substrates, which brings us to the laboratory setting of the 21st century in order to add lipidomics information to this ecological and evolutionary study. In addition, the study of [76] on plant leaves of Nicotiana benthamiana exposed to ω-3 desaturases (ω3Ds; ∆3) demonstrates a large variety of new enzymatic new pathways (Figure 10).
The African savannah Cape buffalo weighs around from 400 to 800 kg (880– 1760 lbs), while the African forest buffalo are much lighter and weigh between 250 and 320 kg (550-705 lbs) [10,48].
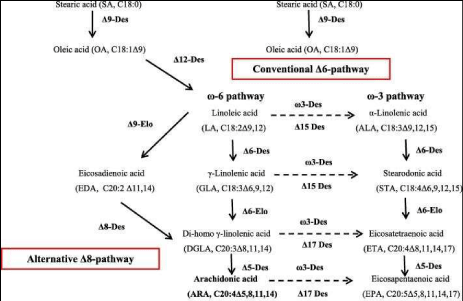
The recent discovery of an alternate ∆8 desaturase pathway
The problem of the reduced amount of synthesized PUFAs via: a). the common ∆6 desaturase routes for the inflammatory ω-6 PUFAs via Linoleic acid (C18:2, ω-6; LA) through restricted ∆6 desaturase activity towards γ-Linolenic acid (C18:3, ω-6; GLA) and conversely b). the non-inflammatory ω-3 PUFAs via α-Linolenic acid (C18:3, ω-3; ALA) by the other ∆6-desaturase route towards Stearidonic acid (C18:4, ω-3; STA), can probably be overcome by a ∆8 desaturase pathway. The recent discovery of an ω-6 inflammatory ∆8 desaturase pathway in mosses like Physcomitrella patens [77] and conversely, an ω-3 non- inflammatory ∆8 desaturase pathway would both have the ability to increase tremendously the amount of LCPUFAs (Figure 10; [78,79].
In nature, multiple fatty acid (FA) substrates are available for ∆3-desaturase as demonstrated for plant leaves of Nicotiana benthamiana. [76] attempted to measure the conversion efficiency of each substrate from a pool of five different substrates: (C18:3, ω6 ALA), Dihomo-ℽ-Linolenic acid (C20:3,ω6; DGLA), Arachidonic acid (C20:4ω6, ARA), Docosatetraenoic acid (C22:4, ω6; DTA) and Docosapentaenoic acid (C22:5,ω6; DPA). Unlike the individual substrate essays, ω3D (∆3-desaturase) showed the highest conversion rate for Arachidonic acid (C20:4, ω-6; ARA), up to 15.2 ± 3.6% among the pool of substrates followed by Docosatetraenoic acid (C22: 4, ω-6; DTA); Osbond acid (C22:5, ω-6; OA); ℽ- Linolenic acid (C18:3,ω-6; GLA) and Dihomo-ℽ-Linolenic acid (C20:3, ω6; DGLA). This study points out to us that Syncerus caffer can use another ecological habitat, the tropical forest ‘biomes’, during the dry season (Figure 10) which has an impact on the composition of PUFAs in the diet, which is affected by the typical enzymatic conversions unfamiliar in human and animal PUFA conversion, which are so characteristic for plant leaves [76].
Linoleic acid (C18:2, ω-6; LA) has important functions in cholesterol metabolism and in specific skin lipids. α-Linolenic acid (C18:3, ω-3; ALA) is not known to serve any essential function other than as a precursor for EPA and DHA. However, a large proportion of α- Linolenic acid (C18:3, ω-3; ALA) is β-oxidized to acetyl CoA in the mitochondrial membrane, which is recycled into cholesterol and into saturated and monounsaturated fatty acids (MUFAs), or further metabolized to CO2. Unlike Linoleic acid (C18:2, ω-6; LA) acylation of α-Linolenic acid (C18:3, ω-3; ALA) in tissue lipids is very low.
It is not yet known whether sufficient Arachidonic acid (C20: 4, ω-6; ARA) enters the desaturation pathway to maintain optimal neural and retinal Docosahexaenoic acid (C22: 6, ω-3; DHA) levels during the development of early human-like brains in young infants and early hominids. Clarification of the regulation of the distribution of Linoleic acid (C18: 2, ω- 6; LA) and α-Linolenic acid (C18: 3, ω-3; ALA) and their potential fate of direct esterification in tissue lipids, β-oxidation and desaturation will be useful to tackle this biochemical research topic [59].
Physiology of the ruminant African Buffalo Syncerus caffer
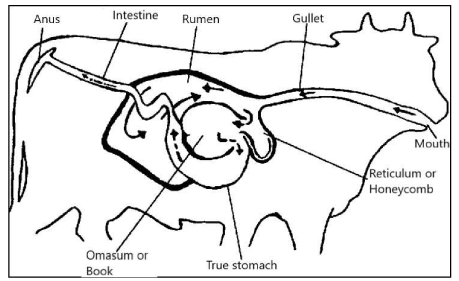
In this review article of evolutionary perception, we are interested in the fatty acid composition of the meat of ruminants -with emphasis on the African buffalo (Syncerus caffer)- because in an earlier review manuscript we proposed, mainly based on ecological grounds, that it may have played a role in encephalization of modern archaic man Homo sapiens at the African savannah [9]. With respect to the digestive tract, ruminants do not have one compartment for the stomach but four: the rumen, the reticulum, the omasum and the true stomach, the abdomen. The rumen is the largest of these and the most important digestive center [Figure 11, 68].
In the rumen of cattle and sheep, it was demonstrated that there was a bacterial abundance of the strains Barnesiella, Prevotella, Paraprevotella, Hallela, Anaerovorax, Succini-clasticum,Ruminococcus and Ruminobacter all of which may be involved in the ruminal response of biohydrogenation to the addition of PUFAs by feed [81]. Microbial PUFA production has been described earlier although for different bacterial strains (Mortierella alpine, Conidiobolus nanodes, Entomophthora exitales, Blastocladiella emersomit) by [82].
The following pathways were given for PUFA conversion by microbial organisms (Figure 13a): Stearic acid (C18:0; ω-9; SA) (C18:0) is converted to Oleic acid (C18:1) by the formation of the first double bond at its Δ9 position; it is then desaturated by Δ12 desaturase to yield Linoleic acid (C18:2, ω-6; LA), which may subsequently be converted by Δl5 desaturase to α-Linolenic acid (C18:3, ω-3; ALA). Thus, Oleic acid, LA, and ALA are basic precursors of ω-9, ω-6, and ω-3 fatty acids. The next steps involve desaturation of fatty acid precursors by Δ6 desaturase, followed by elongations and subsequent desaturation(s) to produce the C20 and C22 PUFAs, respectively. The ω-9 family of PUFAs is synthesized from Oleic acid (C18:1) and sequential participation of Δ6 desaturase, elongase, and Δ5 desaturase to finally produce Mead acid (C20:3, ω-9; MA) [83]. Linoleic acid is converted via Δ6 desaturase and Δ6 elongase steps to ℽ-Linolenic acid (C18:3, ω-6; GLA) and Dihomo-ℽ-Linolenic acid (C20:3, ω-6; DGLA), after which steps involving Δ5 desaturase and Δ5 elongase produce Arachidonic acid (C20:4, ω-6; ARA) and Adrenic acid (C24: 4, ω-6; AA). The final step is catalyzed by Δ5 desaturase to produce Docosapentaenoic acid (C22:5, ω-3; DPA).
A similar pathway with the same enzymes can be found in ω-3 family producing Eicosapentaenoic acid (C20:5, ω-3; EPA) and Docosahexaenoic acid (C22:6, ω-3; DHA). There are two biosynthetic pathways for the production of ω-3 PUFAs in microorganisms [84]. In the first, which is temperature independent, EPA, DPA and DHA are produced via desaturation and elongation of ALA. The second pathway is temperature dependent and includes the conversion of ω-6 fatty acids to ω-3 PUFAs synthesized by two hypothetical enzymes, Δ15 and Δ17 desaturases [85]. Figure 13a is based on PUFA production by microalgae [86] but in principle the various desaturase pathways remain similar for the paunch microbiome [85].
Biosynthetic pathways of polyunsaturated fatty acids in microalgae [86]. An ∆3, ∆4, ∆5, ∆6, ∆8, ∆9, ∆12, ∆15, ∆17 desaturase enzymatic activity can be observed. We assume that for microorganisms in the paunch of Syncerus caffer, the biochemical pathways are similar [85]. One may assume that the rumen microbiome and its PUFA interconversions are to a great extent determined by the catabolized substrates.
These bacteria allow the ruminant to break down grass and other coarse vegetation which animals with one stomach (monogastric, simply mashed; including humans, chickens, zebras and pigs) cannot digest. Ruminants do not chew entirely the grass or the vegetation they eat. The partially chewed grass enters the large rumen, where it is stored and broken into balls named "Cud". When the animal has eaten, it will rest and "chew". The ruminant mass is then swallowed again, after which it ends up in the following three compartments: the reticulum, the omasum and the true stomach, the abdomen Figure 12, [68]. Thus, ruminants can convert low digestible plants and residues into high-quality proteins in the form of meat and milk.
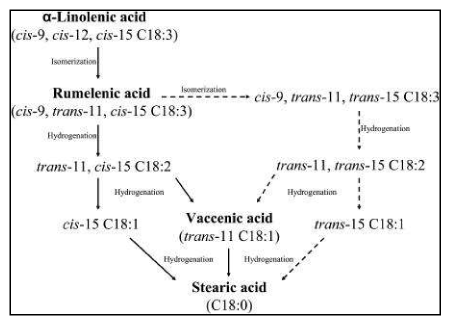
The formation of Rumenic acid (C18:2, ω-7; RA) (Figure 13b) [87] and other different processes in lipid metabolism involving rumen bacteria which occur in ruminants will be exemplified in this paragraph. This process exerts a major influence on the profile of fatty acids (FAs) available for absorption and on the use of the tissue. Unsaturated fatty acids taken in with food by ruminants are converted into Saturated Fatty acids (SFAs) in the process of lipolysis and biohydrogenation. The initial step in the metabolism of dietary lipids which enter the rumen is lipolysis. During this process, the ester linkages of triacylglycerols, phospholipids and galactolipids are hydrolysed by the rumen bacterial enzymes, releasing free fatty acids (FFAs).
Released unsaturated fatty acids consist mainly of Linoleic acid (C18:2, ω-6; LA) and α- Linolenic acid (C18:3, ω-3; ALA). These two Essential Fatty acids (EFAs) are bio- hydrogenated by rumen bacteria, leading to the formation of SFAs. In ruminants, the fatty acid 18:1 t-11 Vaccenic acid (C18:1, ω-7; VA) is an important intermediate produced by microorganisms in the rumen. After its absorption, this fatty acid can be transformed into CLA (18:2 c-9, t-11-Rumenic acid) or Rumenic acid (C18:2, ω-7; RA) in tissues of ruminants [88]. The majority of fatty acids in bull beef consists of Oleic acid (18:1 c-9), Stearic acid (C18:0; ω-9; SA), and Palmitic acid (16:0). As ruminant diets contain low concentrations of fat, the majority of the adipose tissue is formed from de novo lipogenesis. Fatty acids are elongated to form Stearic acid (C18:0; ω-9; SA) and converted into Oleic acid (C18:1) by ∆9 desaturase [89]. As the formation of adipose tissue increases, the high deposition of Oleic acid (C18:1) reduces the Stearic acid (C18:0; ω-9; SA) content.
The fate of the two Essential Fatty acids (EFAs) via isomerization and reduction via Rumenic acid (C18:2, ω-7; RA) [87] and Vaccenic acid (C18:1, ω-7; VA) towards Stearic acid (C18:0; ω-9; SA) for the ω-3 EFA α-Linolenic acid (C18:3, ω-3; ALA). While for the ω-6 EFA Linoleic acid (C18:2, ω-6; LA) an isomerization leads towards Rumenic acid (C18:2, ω-7; RA) and finally via reductase reactions towards also Stearic acid (C18:0; ω-9; SA) [90,91].
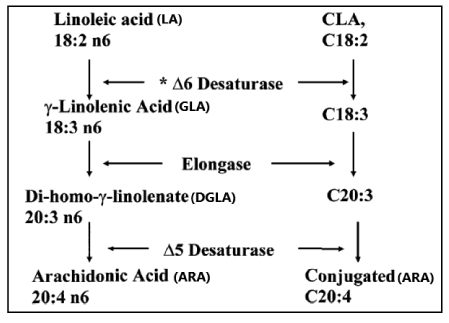
In the formation of Conjugated Linoleic acid (CLA), the conversion by ∆6 desaturase is the rate limiting step (Figure 14; [92].
Pathway for desaturation and elongation of Conjugated Linoleic acid (CLA). The conversion by ∆6 Desaturase is in the PUFA schedule the rate limiting step in the formation of Arachidonic acid (C20:4, ω-6; ARA) [92].
Ruminal bacteria are involved in biohydrogenation which have been classified into two groups: A and B, based on their metabolic pathways:
Group A: includes bacteria that can hydrogenate PUFAs into trans-11 Oleic acid (C18:1, ω- 9; OA).
Group B: are bacteria that can hydrogenate Oleic acid (C18:1, ω-9) and its isomers as well as trans-11 (C18:1) fatty acids (FAs) to form Stearic acid (C18:0; ω-9; SA).
In order to complete the biohydrogenation of PUFA, the presence of both bacterial groups is required [65]. Thanks to the bacterial activity of ruminants, the PUFA content in meat and milk is significantly increased. A second reinforcing mechanism is the protective mechanism of these essential PUFAs by 'incapsulation' against the catabolic biohydrogenation process of rumen bacteria that can degrade these essential PUFAs [96].
Old problem of limited ∆6 desaturation routes
So, this new historical discovery could reduce the old problem of limited ∆6 desaturation routes for aforementioned a). Linoleic acid (C18: 2, ω 6; LA); b). ℽ-Linolenic acid (C18: 3, ω-6; GLA) and Stearidonic acid (C18: 4, ω- 3; STA) enzymatic activity (Figure 8). This is enhanced by functional studies in mice, with selective ∆-5 and ∆-6 desaturase inhibitors showing clear anti-inflammatory effects. Regulation mechanisms of ∆-5 and ∆-6 desaturases have hardly been investigated in human tissue. The human ∆-5 and ∆-6 desaturase complementary DNAs were first cloned in 1999 [93]. In 2000, they were identified as FADS1 and FADS2 in the human genome [94]. Both genes, FADS1 and FADS2, are oriented head-to- head and located in a cluster on chromosome 11 (11q12-13.1) [79]. In addition, [78] demonstrated that the FADS2 gene product exhibits
∆8 desaturase activity and that it is able to convert the inflammatory Eicosadienoic acid (C20: 2, ω-6; EDA) into Dihomo-γ-Linolenic acid (C20: 3, ω-6; DGLA) and the non - inflammatory Eicosatrienoic acid (C20: 3, ω-3; ETrA) into the non-inflammatory product Eicosatetraenoic acid (C20: 4, ω-3; ETA). Dihomo-γ-Linolenic acid (C20: 3, ω-6; DGLA) is the direct precursor of prostaglandin E1 and thromboxane B1. DGLA and Eicosatetraenoic acid (C20: 4, ω-3; ETA) are also direct precursors of long chain polyunsaturated fatty acids (LC-PUFAs) Arachidonic acid (C20: 4, ω-6; ARA) and Eicosapentaenoic acid (C20: 5, ω-3; EPA) respectively the latter of which is essential for the human brain. These findings provide unambiguous molecular evidence for a new alternative biosynthetic route for the formation of long-chain polyunsaturated fatty acids (PUFAs) in mammals from substrates that were previously considered dead-end products [78]. Finally, after leaving the rumen, the FFAs from the particles of food and bacteria are desorbed by lysolecithin and bile salts. The micelles formed in this process are taken up by the epithelial cells of the jejunum. The free fatty acids are released from the small amounts of triacylglycerols and glycolipids reaching the intestines [65]. Subsequently, lipids are converted to meat and lard of Syncerus caffer.
The first stage in biohydrogenation of Linoleic fatty acids (C18:2, ω-6; LA) involves saturation which consists of isomerization reactions. During the isomerization reactions Linoleic acid (C18:2, ω-6; LA) is converted into the isomer cis-9, trans-11 conjugated Linoleic acid (CLA, Rumenic acid (C18:2, ω-7; RA) [87] which is subsequently reduced to Vaccenic acid (C18:1, ω-7; VA) [96], a monoenoic fatty acid. In the next stage Vaccenic acid (C18:1, ω-7; VA) is converted into Stearic acid (C18:0; ω-9; SA) (Figure 13).
α-Linolenic acid (C18:3, ω-3; ALA) is biohydrogenated following the same pattern starting with an isomerization reaction to form Rumenic acid (C18:2, ω-7; RA), followed by a sequence of reductions and completed with the formation of Stearic acid (C18:0; ω-9; SA) (Figure 13: [73,91,96]. Vaccenic acid (C18:1, ω-7; VA) (Figure 13) and Conjugated Linoleic acid (CLA) (Figure 14 & Figure 18) are the most important intermediaries formed during these transformations [65]. VA is a substrate for the production of CLA cis-9, trans-11 (C18:2) which is the main CLA isomer in beef and is mainly associated with the triacyl- glycerol lipid fraction and therefore it correlates positively with the level of fatness [96]. In tissues, Δ-9 desaturate converts Stearic acid (C18:0; ω-9; SA) into cis- 9 Oleic acid (C18:1).
Mammals lack Δ12 and Δ15-desaturase activities (Figure 15), so they cannot synthesize Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA) from the precursor Oleic acid (18:1, ω-9; OA) [97]. Linoleic acid (C18:2, ω-6; LA) and
α-Linolenic acid (C18:3, ω-3; ALA) are therefore considered essential dietary nutrients and designated essential fatty acids (EFAs). The metabolism of the production of PUFAs from these two essential fatty acids (EFAs) proceeds via a series of elongation and desaturation steps which involves insertion of additional double bonds into the aliphatic chain (a process termed desaturation) and addition of further carbon atoms to the acyl chain (a process termed elongation).
Essential fatty acids are important components of structural lipids and contribute to the regulation of membrane properties such as fluidity, flexibility, permeability and modulation of membrane-bound proteins. Linoleic acid (LA, 18:2ω6) and α-linolenic acid (ALA, 18:3ω3) are the two parent EFAs (Figure 8).
The term 'essential' implies that they must be supplied in the diet because they are required by the human body but cannot be endogenously synthesised. The balance between ω3- and ω6- FAs in the diet is important because of their competitive nature and their different biological roles. Both parent EFAs are metabolised to long chain polyunsaturated fatty acids (LC- PUFAs) of 20- and 22- carbon atoms. EFAs and LC-PUFAs may together be referred to as polyunsaturated fatty acids (PUFAs). Once obtained from the diet, Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA) are further metabolized by ∆-6 desaturation, elongation, and ∆-5 desaturation to form Arachidonic acid (C20:4, ω-6; ARA) and Eicosapentaenoic acid (C:20:5, ω–3, EPA) respectively (Figure 8). The ∆5-desaturase catalysed and subsequent steps in the pathway occur in mammals but not in plant cells (Figure 15).
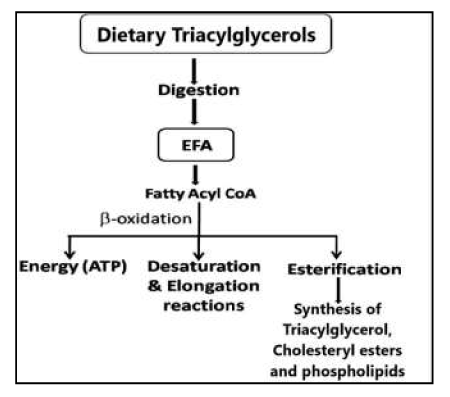
Biochemical composition of the meat of the African buffalo (Syncerus caffer)The ω-6 and ω-3 fatty acids when consumed in the diet in the form of triacylglycerols from various food sources undergo digestion in the small intestine which allows absorption and transport in the blood and subsequent assimilation in the body including brain, retina, heart and other tissues. These fatty acids can undergo:
- β-oxidation to provide energy in the form of ATP;
- Esterification into cellular lipids including triacylglycerols, cholesteryl esters (ChE) and phospholipids;
(c) Conversion into their important longer chain and more highly unsaturated products by a series of desaturation and elongation reactions actively taking place particularly in the liver and to a lesser extent in other tissues Figure 17, [98].
Dietary fat is ingested principally in the form of triacylglycerols containing the fatty acids Palmitic acid (C16:0, ω-7; PA) and Oleic acid (C18:1, ω-9; OA) [99]. As mentioned before, mammals lack Δ12 and Δ15-desaturase activities (Figure 15), hence cannot synthesize Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA) from the precursor Oleic acid (18:1, ω-9; OA) [97].
Dietary triacylglycerols of animal origin predominantly contain long-chain (i.e., longer than C14 chain length) saturated fatty acids. Digestion and absorption of dietary fat is generally completed by the middle third of jejunum.
The liver plays a central role in controlling various aspects of fat metabolism. For a detailed description of the various processes, consult the excellent review of [100].
In this review, we try to present and combine as much physiological and biochemical information as possible relating to the African Buffalo meat and lard composition and describe its relevance for human brain growth (encephalization). One piece of such important information concerns the biochemical muscle composition of Syncerus caffer in comparison with Cod liver oil (Table 2).
Table 2: Percentage of Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA) elongase-products of total fatty acid in muscle tissue from Syncerus caffer (n=7) in comparison with a fish oil (Cod liver oil)
|
Fatty acid assignment |
Positions of double bonds |
Extracted fatty acids Mean ± STD (n=7) (Percentage composition) (20. Crawford et al 1969) |
|
Cod liver oil (Percentage composition) (101. Ackman & Burgher 1964) |
|
Linoleic acid (C18:2, ω-6; LA) |
9, 12 |
17.3 ± 1.1 |
|
0.8 |
|
α-Linolenic acid (C18:3, ω-3; ALA) |
9, 12, 15 |
5.4 ± 0.8 |
|
0.1 |
|
Eicosadienoic acid (C20:2, ω-6; EDA) |
11, 14 |
0.27 ± 0.07 |
|
0.2 |
|
Eicosatrienoic acid (C20:3, ω-3; ETrA) |
8, 11, 114 |
0.42 ± 0.14 |
|
0.1 |
|
Arachidonic acid (C20:4, ω-6; ARA) |
5, 8, 11, 14 |
7.5 ± 0.67 |
|
1.2 |
|
Eicosatetraenoic acid (C20:4, ω-3; ETA) |
8, 11, 14, 17 |
0.3 ± 0.06 |
|
0.5 |
|
Eicosapentaenoic acid (C20:5, ω-3; EPA) |
5, 8, 11, 14, 17 |
1.5 ± 0.31 |
|
5.0 |
|
Docosapentaenoic acid (C22:5, ω-3; DPA) |
7, 10, 13, 16, 19 |
3.0 ± 0.18 |
|
1.9 |
|
Docosahexaenoic acid (C22:6, ω-3; DHA) |
4, 7, 10, 13, 16, 19 |
0.26 ± 0.05 |
|
10.5 |
|
Total ω6 Acids |
25 |
- |
|
2.3 |
|
Total ω3 Acids |
10 |
- |
|
18 |
Ruminant meat fat comprises mostly monounsaturated fatty acids (MUFAs) and saturated fatty acids (SFAs). The most ubiquitous fatty acids are oleic (C18:1), palmitic (C16:0), and stearic (C18:0) acids. The major PUFA in ruminants -so important for a healthy brain and probably also for the process of human brain encephalization- is Linoleic acid (C18:2, ω-6; LA; (0.5–7%) followed by α-Linolenic acid (C18:3, ω-3; ALA; up to 0.5%) [57].
If we compare these values with Table 1, we see that Syncerus caffer has exceptionally high values for both EFAs, around 17.3% for Linoleic acid (C18:2, ω-6; LA), around 5.4% for α- Linolenic acid (C18:3, ω-3; ALA) and, in addition, around 7.5% for Arachidonic acid (C20:4, ω-6, ARA). Of the C18 fatty acids (EFAs) in the elongase-desaturase array, Linoleic acid (C18: 2, ω6; LA) is the most common in the muscle tissue [20].
Trans-fatty acids comprise about 1–2% of total fatty acids across all types of meat; in ruminant meat they represent ≈2–4%.
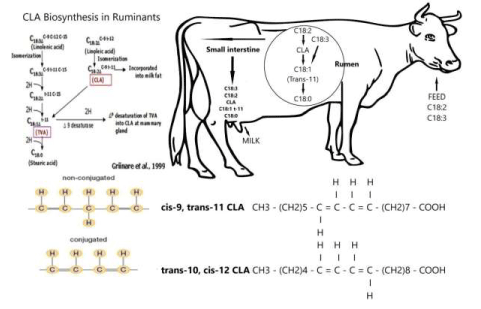
Conjugated linoleic acid (CLA) refers to a group of polyunsaturated fatty acids that exist as positional and stereoisomers of a conjugated dienoic octadecadienoate (C18:2) (Figure 18). At the American Oil Chemists’ Society meeting in Chicago, May 10–13, 1998, a formal discussion at a plenary slot was devoted to the naming of the major Conjugated Linoleic acid isomer (C18:2, ω-6; CLA) isomer, cis-9, trans-11-octadecadienoic acid which is found in natural dairy products like milk and meats of ruminant animals. In Figure 19, its interconversions between rumen and tissues are depicted. The result of this discussion was an unchanged name, but most importantly it emphasized the importance of ‘Rumenic acid’ (C18:2, ω-7; RA) for the human food industry based on dairy products [87] Interactions of the various FAs in the rumen compartment and in tissues.
Most important is the conversion, in the rumen, of Linoleic Acid (C18:2, ω-6; LA) via CLA into Stearic acid (C18:0; ω-9; SA) and that of the latter via ∆9 desaturase activity into Oleic acid (C18:1) and ultimately to Linoleic Acid (C18:2, ω-6; LA) which is the most common in muscle tissues of Syncerus caffer [20].
CLA is an intermediate in the biohydrogenation and partly escapes the rumen after which it is absorbed in milk fat and body fat (Figure 19). In addition, the animal itself synthesizes cis-9, trans-11 CLA from trans-11 octadecenoic acid, another intermediate of rumen biohydrogenation which is absorbed. This conversion involves ∆9 desaturase which is present in breast tissue (lactation) and adipose tissue (growth). The cis-9, trans-11 CLA isomer is the major isomer found in ruminant fat; this isomer typically represents 80 to 90% of the total CLA in milk fat, but its occurrence in beef fat is less. Under certain feeding conditions, the proportion of the trans-10, cis-12 CLA isomer increases. Dietary factors therefore also change the direction of the biohydrogenation pathways in the rumen [103].
Table 3 presents the PUFAs measured in the muscle of the current African Buffalo (Syncerus caffer) in comparison to Cod liver oil and the enzymatic activities involved in their conversions, cautiously calculated based on product/precursor ratios as reported earlier [104].
Table 3: This Table 3 is an extension of Table 2 with an extension of the enzyme activities based on the calculation of product precursor ratios for muscle tissue from Syncerus caffer (n=7) in comparison with a fish oil (Cod liver oil)
|
Fatty acid assignment |
Positions of double bonds |
Extracted fatty acids Mean ± STD (n=7) (Percentage composition) (20. Crawford et al 1969) |
Cod liver oil (Percentage composition) (101. Ackman & Burgher 1964) |
|
Linoleic acid (C18:2, ω-6; LA) |
9, 12 |
17.3 ± 1.1 |
0.8 |
|
∆9 elongase (ω-6 side) |
- |
0.27/17.3=0.27 |
0.2/0.8 |
|
Eicosadienoic acid (C20:2, ω-6; EDA) |
11, 14 |
0.27 ± 0.07 |
0.2 |
|
∆8 desaturase + ∆5 desaturase activity |
- |
7.5/0.27=27.8 |
1.2/0.2 |
|
Arachidonic acid (C20:4, ω-6; ARA) |
5, 8, 11, 14 |
7.5 ± 0.67 |
1.2 |
|
∆17 desaturase |
- |
1.5/7.5=0.2 |
5.0/1.2 |
|
Eicosapentaenoic acid (C20:5, ω-3; EPA) |
5, 8, 11, 14, 17 |
1.5 ± 0.31 |
5.0 |
|
α-Linolenic acid (C18:3, ω-3; ALA) |
9, 12, 15 |
5.4 ± 0.8 |
0.1 |
|
∆9 elongase (ω-3 side) |
- |
0.42/5.4=0.078 |
0.1/0.1 |
|
Eicosatrienoic acid (C20:3, ω-3; ETrA) |
8, 11, 114 |
0.42 ± 0.14 |
0.1 |
|
∆8 desaturase |
|
0.3/0.42=0.71 |
0.5/0.1 |
|
Eicosatetraenoic acid (C20:4, ω-3; ETA) |
8, 11, 14, 17 |
0.3 ± 0.06 |
0.5 |
|
∆5 desaturase |
- |
1.5/0.3=5.0 |
5.0/0.5 |
|
Eicosapentaenoic acid (C20:5, ω-3; EPA) |
5, 8, 11, 14, 17 |
1.5 ± 0.31 |
5.0 |
|
C20/22 elongase |
- |
3.0/1.5=2 |
1.9/5.0 |
|
Docosapentaenoic acid (C22:5, ω-3; DPA) |
7, 10, 13, 16, 19 |
3.0 ± 0.18 |
1.9 |
|
∆4 desaturase= peroxisomal Sprecher’s shunt |
- |
0.26/3.0=0.087 |
10.5/1.9 |
|
Docosahexaenoic acid (C22:6, ω-3; DHA) |
4, 7, 10, 13, 16, 19 |
0.26 ± 0.05 |
10.5 |
|
Total ω6 Acids |
25 |
|
2.3 |
|
Total ω3 Acids |
10 |
|
18 |
From Table 3 we can cautiously conclude the occurrence of ∆9 elongase-, ∆17 desaturase-; ∆9 elongase; ∆8 desaturase, ∆5 desaturase and ∆4 desaturase (≈Sprecher’s shunt) enzymatic activity. If we relate these observations to Figure 15, we observe that it is generally acknowledged that mammals exhibit ∆5-, ∆6-, and ∆9- desaturase activity [45].
The observed ∆17-desaturase, ∆8-desaturase and ∆4-desaturase (partly incorporated in Sprecher’s shunt) activity, however, is very unusual and may lay at the basis of the typically observed fatty acid composition of meat with extremely high concentrations of Arachidonic acid (C20:4, ω-6; ARA) and Docosapentaenoic acid (C22:5, ω-3; DPA). Because the African buffalo (Syncerus caffer) is a real ruminant, it is unlikely that it has access to polyenoic acids higher than Linoleic acid (C18: 2, ω6; LA) and α-Linolenic acid (C18: 3, ω3; ALA) in its food. This also agrees with the earlier described GCMS measurements performed on the most important African savannah grass Andropogon gayanus serving as feed for Syncerus caffer for which TAGs were mainly composed of P2O, P2L, PO2, POL, O3, PL2, O2L, L2O, and L3 with P=Palmitic acid (C16:0; PA), O=Oleic acid (C18:1; OA) and L=Linoleic acid (C18:2, ω-6; LA) [70]. So, two important conclusions can be drawn. First, all combinations of the aforementioned fatty acids linked to the glycerol backbone molecule can be found. Secondly, the major EFA is an inflammatory one, ω-6, Linoleic acid (C18:2, ω- 6; LA). The source of C20 polyenoic acids and longer chain length like ARA and DPA most likely consists of animal metabolism via the earlier described PUFA conversion routes.
Arachidonic acid (C20:4, ω-6; ARA)Higher intakes of Arachidonic acid (C20:4, ω-6; ARA) and a much lower ratio of [LA: ARA] is one strategy for moderately enhancing the conversion of ARA to EPA [105]. However, many human studies in which the ratio of [LA: ARA] was lowered, have not shown a significant rise in DHA, not even with a higher intake of ARA despite the moderate rise in EPA [105].
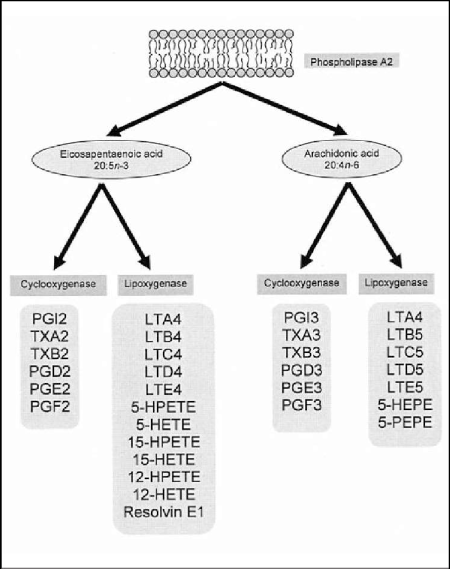
Balance between omega-6 PUFAs (≈Proinflammatory mediators) derived from Arachidonic acid (C20:4, ω-6; ARA) and omega-3 PUFAs which produce anti-inflammatory and protective compounds (Resolvins and Neuroprotectins) derived from Docosahexaenoic acid (C22:6, ω-3, DHA).
Dietary fat composition influences eicosanoid synthesis by changing the supply of substrates for the synthesis of the longer-chain ω-3 and ω-6 PUFA. Consuming large amounts of Linoleic acid (C18:2, ω-6; LA) increases the quantity of Arachidonic acid (C20:4, ω-6; ARA) in cell membranes. Upon activation, in a variety of cells, Arachidonic acid is converted to eicosanoids of the 2-series and leukotrienes of the 4-series (Figure 20). However, dietary α- Linolenic acid is converted to Eicosapentaenoic acid (20:5n-3; EPA) in the membrane, and when cells are activated, Eicosapentaenoic acid (20:5n-3; EPA) is converted to eicosanoids of the 3-series and leukotrienes of the 5-series (Figure 20). In general, eicosanoids formed from ω-3 PUFA oppose, or have weaker effects than eicosanoids formed from ω-6 PUFA. ω-3 fatty acids inhibit Δ-6 and Δ-5 desaturase activity, reducing the synthesis of Arachidonic acid (C20:4, ω-6; ARA) in the membrane [106] Indeed, it has been shown that increasing the proportions of ω-3 in relation to ω-6 PUFAs in the diet decreases the quantity of proinflammatory, vasoconstrictive, platelet aggregatory, and immunosuppressive compounds that are regulated by eicosanoids originating from Arachidonic acid (C20:4, ω-6; ARA). Recent investigations have shown that Dihomo-γ-Linolenic acid (C20:3, ω-6 DGLA) present in some plants (i.e. savannah seed, evening primrose oil, borage, and flaxseed) may also influence the type of eicosanoid synthesized by a cell.
Ω-3 Docosapentaenoic acid (C22:5, ω-3; DPA)
Because the evolution of the human brain can largely be compared with the embryological development of the brain of the fetus during pregnancy, we are interested from an evolutionary point of view (with biochemical perception) in the comparable ontogeny of the different stages of development. In this respect, ω-3 DPA is indeed the second ω-3 LC-PUFA found in the brain (w / w), although the cerebral concentration is approximately 70 times lower than DHA. In addition, the level of ω- 3 DPA in breast milk is higher than that of EPA, similar to that of DHA and the level is more sTable [107], which is a possible effect of ω-3 DPA during pregnancy and development as a recent study suggests [108]. In addition, many studies have therefore attempted to increase neurocognitive results in infants through (maternal) ω-3 LCPUFA supplementation. However, it seems unlikely that differences between the LCPUFA composition in breast milk and only the formula could explain the beneficial effects of breastfeeding on infant nutrition and children's health and the results of neurological development (see also excellent assessment of (109. Schipper et al. 2020) on this topic).
Although obtaining optimal tissue status in ω-3 LC-PUFA is one of the current public health challenges, ω-3 DPA is also the only intermediate between EPA and DHA in the ω-3 LC- PUFA conversion range of α- Linolenic acid (ALA) present in substantial amounts in the diet.
Bioconversion pathway of ω-3 and ω-6 polyunsaturated fatty acids (PUFAs) families [75] ∆: desaturase; ε: elongase; ALA: α-Linolenic acid; ADA: Adrenic acid; ARA: Arachidonic acid; EPA: Eicosapentaenoic acid; GLA: ℽ-Linolenic acid; DHA: Docosahexaenoic acid; ω-3 DPA: ω-3 Docosapentaenoic acid; LA: Linoleic acid; STA: Stearidonic acid.
Could ω-3 DPA therefore serve as a food source or biological reservoir for DHA and EPA? ω-3 DPA is the direct intermediate between EPA and DHA in the ALA conversion path, and present in significant amounts in the human diet compared to the C24: 5 ω-3 and C24: 6 ω-3 derivatives [110]. This conversion route is well known and includes a series of desaturase enzymes that add one double bond to the carbon chain and of elongase enzymes that lengthen the carbon chain with two carbon atoms (Figure 21). The ω-3 LCPUFA conversion route is parallel to that of ω-6 LCPUFA with the same sequence and enzymes. The two paths for substrates therefore compete with each other. In the ω-3 LCPUFA conversion route, the n-3 DPA is extended to the Tetracosapentaenoic acid (C24:5 ω-3; TPA) derivative by the elongase-5 and the weaker elongase-2 enzymes, then desaturated to the Tetracosahexaenoic acid (C24:6, ω-3) by the action of the ∆6 desaturase and eventually converted to DHA by a peroxisomal β-oxidation step [111]. The ∆6 desaturase and more recently characterized elongase-2 are considered to be the limiting enzymes in this conversion path to DHA [111]. Therefore, it is hypothesized that ω-3 DPA in the diet could be a more effective precursor of DHA than EPA whereby the conversion from EPA to ω-3 DPA is also bypassed through the activity of elongase-2 and elongase-5 [75]. The apparent retro- conversion of ω-3 DPA to EPA was demonstrated in humans [112], the Sprague-Dawley rat [113], the C57BL/6J-db/db mouse [114], the C57BL/6J mouse (high-fat diet) [112] and in miniature poodle dogs [115]. Labeled ω-3 DPA monitoring studies need to confirm and quantify the actual presence of this pathway [75].
One ∆4 desaturase activity has also been described in vitro in humans converting ω-3 Docosapentaenoic acid (C22:5, ω-3; DPA) directly into Docosahexaeinoic acid (C22:6, ω-3; DHA), but these results need to be confirmed [116]. Some authors also hypothesize that ω-3 Docosapentaenoic acid (C22:5, ω-3; DPA) can serve not only as a reservoir of DHA but also of EPA in humans, in farm animals and possibly in other mammals [Figures 22, 117].
Synthesis of Eicosapentaenoic acid (C20:5, ω-3; EPA), Docosapentaenoic acid (C22:5, ω-3; DPA) and Docosahexaeinoic acid (C22:6, ω-3; DHA) from Arachidonic acid (C20:4, ω-6; ARA). The longer chain omega-3 polyunsaturated fatty acids (PUFAs) are synthesized from ARA by a progressive series of enzymatic desaturation and chain elongation steps, initially in the endoplasmic reticulum. In the final stage Tetracosahexaenoic acid (C24:6, ω-3) is translocated to the peroxisome and shortened by one cycle of the β- oxidation pathway to form Docosahexaeinoic acid (C22:6, ω-3; DHA). It has been suggested that Docosapentaenoic acid (C22:5, ω-3; DPA) is possibly a buffer between the two important ω-3 PUFAs Eicosapentaenoic acid (C20:5, ω-3; EPA) and Docosahexaeinoic acid (C22:6, ω-3; DHA) [75].
Digestion and absorption of fats in the diet by humansDigestion and absorption of fats in the diet, including those in beef, depend to some extent on the size and degree of unsaturation of the fatty acids and on the structure of the fat molecules which implies the position of different fatty acids in the lipid molecule [118].
Digestion of fats in humans is accomplished by lipase enzymes that hydrolyse the ester bonds, releasing the fatty acids from glycerol. In order to study this topic, intragastric fat digestion was investigated by analysing the products of lipolysis and the gastric lipase (HGL) levels of premature infants fed with a formula enriched with Medium Chain Triacylglycerols (MCT) and those of infants fed with human milk. The major outcome of this study was that the main fatty acids released in the stomach in both groups were Palmitic acid (C16:0 around 17.0%) and Oleic acid (C18:1 around 28.2%) [99]. As mentioned earlier (Figure 15; [45] mammals lack Δ12 and Δ15-desaturase activities, so they cannot synthesize Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA) from the precursor Oleic acid (18:1, ω-9; OA) [97].
The structural organization of fatty acids in food and in the body is mainly determined by their binding to glycerol through ester bonds. The reaction of a hydroxyl group of glycerol, in one of its three positions, with a fatty acid leads to a monoacylglycerol. The coupling of a second fatty acid, which may be similar or different to the first, gives rise to a diacylglycerol. If all three hydroxyl groups of glycerol are linked to fatty acids, then this is a triacylglycerol (Figure 23).
In the small intestine, ’pancreatic lipase’ catalyses the hydrolysis of dietary triacylglycerols to 2-monoacylglycerols by removing fatty acids from the sn-1 and sn-3 positions. The 2- monoacylglycerol molecule is further hydrolysed to release the last fatty acid from the sn-2 position [119].
[120] describe in an important review article how several FAs at the glycerol backbone molecule (Figure 23) behave in the intestine of the rat. The major observation of their research was that the fatty acid species and the location of fatty acids within TAG molecules could significantly affect lipid digestion fates in the gastrointestinal tract, with short chain saturated fatty acids located at the sn-1, 3 position favouring the lipid digestion process [120]. Next, in the stomach, the ‘lingual lipase’ removes the #3 fatty acid from triacylglycerols (TGs). Liberated short-chain fatty acids (<12 C) are soluble and are absorbed through the stomach mucosa into the portal vein, which carries them to the liver where they are metabolized to release energy [121]. Lipolysis is defined as the catabolism of TGs stored in cellular lipid droplets. Recent discoveries of essential lipolytic enzymes and characterization of numerous regulatory proteins and mechanisms have fundamentally changed our perception of lipolysis and its impact on cellular metabolism. New findings, notably that lipolytic products and intermediates participate in cellular signalling processes and that “lipolytic signalling” is particularly important in many non- adipose tissues, unveil a previously underappreciated aspect of lipolysis which may be relevant for the process of “brain steatosis” via LC-PUFAs.
Liberated long-chain fatty acids diffuse (LCFAs) into fat droplets and pass into the small intestine along with the remaining diacylglycerol molecules that contain two fatty acids [122]. In the small intestine ‘pancreatic lipase’ removes the #1 and any remaining #3 fatty acids, leaving a monoacylglycerol with only the #2 fatty acid. Short-
chain fatty acids can be absorbed directly from the intestine and transported to the liver. Long-chain saturated fatty acids (>18 C) tend to form insoluble hydrated acid soaps in the less acidic environment of the small intestine, particularly when calcium and magnesium are present. These insoluble complexes are usually eliminated with the faecal matter. Medium- chain fatty acids may be absorbed or eliminated depending on acidity and availability of minerals that may form complexes. The monoacylglycerol attaches to two other free fatty acids and this triacylglycerol enters the lymphatic system and is transported to the liver and other parts of the body like the cardiac muscle [123], or in our studies with the High-fat diet induced obese C57bl6 mouse model, to the brain [12].
A lipase in the liver may subsequently split off fatty acids from these molecules. Absorption of fatty acids depends on their solubility (which is greater for unsaturated fatty acids and shorter chain saturated fatty acids) and their position on the glycerol backbone.
When Stearic acid (C18:0; ω-9; SA) is in the #2 position (oleic–stearic–oleic), >97% of it is absorbed; when it is in the #1 and 3 positions (stearic–oleic–stearic), only 37% is absorbed. However, if the rats used in the experiment were deficient in calcium and magnesium, 70% of the Stearic acid (C18:0; ω-9; SA) in the #1 and #3 positions was absorbed. In addition to the formation of insoluble soaps, it is likely that some of the Stearic acid containing diacylglycerols, formed after the first lipase reaction, were also eliminated because they were less digestible [121,124,125].
In addition, some recent post-prandial studies in humans [126-129] researchers have investigated structural TAG influences on digestion, absorption and transport or molecular TAG molecules that rise from C16: 0-rich, C18: 1-rich and C18: 0-rich fats, for which the literature has been extensively assessed by [118] The stereospecificity of fatty acids in TAGs is characteristic of natural oils and fats as indicated in Table 4 [130-133]. TAG molecules that make up animal fatty tissue mostly have a saturated fatty acid (SFA) at the sn-1 position and an unsaturated fatty acid at the sn-2 position [132-135]. For example, in beef fat, C16: 0 is in sn-1 position and C18: 1 is in sn-2 position as seen in POO, POP, and POS TAGs but variety among fatty acids is found for the sn-3 position. In contrast, C16: 0 in butterfat is not exclusively linked to sn-1 but occupies sn-2 in two-thirds of TAG types as seen for PPB, PPC and PPO TAGs. In lard, Palmitic acid (C16: 0, ω-7; PA) is found only at the sn-2 position, with an unsaturated fatty acid at sn-3, but the fatty acid that takes the sn-1 position is quite variable, such as in SPO, OPL and OPO TAGs [130]. Oleic acid is common in TAG species of animal or vegeTable origin, usually found at the sn-2 position as observed in olive oil, beef fat, cocoa butter, palm oil, peanut oil and canola oil, but exclusively linked to the sn - 1 and sn - 3 positions only in lard [131,134]; see Table 4.
Table 4: For fatty acid abbreviations in TAG species read as: P = palmitic (C16:0, ω-7); O = oleic (C18:1, ω-9); S = stearic (C18:0, ω-9); C = capric (C10:0, ω-7); L = linoleic (C18:2, ω- 6); B = butyric (C:4, ω-7) (Table modified from: [118].
Table 4: For fatty acid abbreviations in TAG species read as: P = palmitic (C16:0, ω-7); O = oleic (C18:1, ω-9); S = stearic (C18:0, ω-9); C = capric (C10:0, ω-7); L = linoleic (C18:2, ω- 6); B = butyric (C:4, ω-7) (table modified from: (118. Karupaiah & Sundram 2007).
|
Animal Fat |
1 |
1 |
1 |
2 |
2 |
2 |
3 |
3 |
3 |
|
Butter |
palmitic |
Palmitic |
Butyric |
palmitic |
palmitic |
Capric |
palmitic |
Oleic |
Palmitic |
|
Lard |
Stearic |
Palmitic |
Oleic |
Oleic |
palmitic |
Linoleic |
Oleic |
Palmitic |
Oleic |
|
Tallow |
palmitic |
Oleic |
Oleic |
palmitic |
Oleic |
palmitic |
palmitic |
Oleic |
Stearic |
For our model in which such a central role is assigned to bovine lard, it is important to notice that bovine lard (Table 4) has a higher portion of ≈40% palmitic acid (C16:0) in the sn-2 position, which explains its high absorption rate. ‘Lard’ has a very characteristic composition of each main fatty acid in the sn-2 position of the triacylglycerol in comparison to other animal fats (butter & tallow), namely with Palmitic acid (C16:0, ω-7; PA) at the sn-2 position of every combination (1 or 2 or 3; see Table 4; [118,124].
Other items in the diet, including sources of calcium, magnesium, and dietary fibres may also affect fat absorption [57]. Saturated fatty acids (SFAs) may be catabolized for energy, incorporated directly into membranes or into other lipids such as circulating lipoproteins, or may be deposited as stored fat in various locations in the body. In addition, desaturation enzymes can create double bonds in saturated fatty acids, and the resulting unsaturated fatty acids may be used or stored in the body [119,136]. To some extent, fatty acid composition of cell membranes and serum lipids reflects the dietary intake of these fatty acids. But the relationship is not exact because of the complex set of reactions occurring during lipid metabolism and the effects of other dietary components on the physiological fate of ingested lipids. Although reduction of dietary fat to approximately 26% of energy intake appears to have positive effects on serum lipids, more severe reductions in fat intake have been reported to increase plasma triacylglycerol levels and increase synthesis of Palmitic acid (C16:0, ω-7; PA) in the body [137].
In vitro studies with adipocytes suggest the possibility that fatty acid transport proteins may have different affinities for different fatty acids, depending on chain length [138]. While the focus of the review of [139] was on heart and skeletal muscle, the concepts outlined for these tissues in general presumably apply to all tissues with an active fatty acid metabolism including the brain. FATP1 is expressed in many tissues, kidney, lung, skin, adipose tissue, heart, and skeletal muscle [139].
On the evolution of the human brain
In this section, we will elucidate to some extent the role in human physiology and (brain) metabolism of the two important “fish oils” Eicosapentaenoic acid (C20:5, ω-3, EPA) and the ‘invisible’ important “fish oil” Docosahexaenoic acid (C22:6, ω-3; DHA) which are both of extreme importance in understanding human brain ‘encephalization’. Recent fascinating discoveries about DHA’s interconversions and the role of the peroxisome brings us still closer to one innovative and comprehensive evolutionary theory about the human brain growth or encephalization as a replenishment to Darwin's "On the Origin or Species ([25])"- where Charles Darwin neglected to give a plausible explanation for the tremendous rapid growth spurt of the human brain over the last 75,000 years (Figure 2). He circumvented this scientific challenge by the following phrase: “In the distant future I see open fields for far more important researchers. Psychology will be based on a new foundation, that of the necessary acquirements of each mental power and capacity by gradation. Light will be thrown on the origin of man and his history” [25].
PUFAs from dietary food are essential for the development of the early humanoid brain and the function of early humanoids during the course of evolution. Nearly 60% of the dry weight of the human brain consists of lipids [140] of which around 30% are PUFAs [141] and about 35% of the lipids in the gray matter are LCPUFA [142]). In the neuronal membrane, the LCPUFAs support physical and functional membrane properties. Longer chain LCPUFAs increase the fluidity of the membrane due to their wider structure and influence the membrane receptor, enzyme activities and neuronal plasticity [143]. The most common LCPUFAs in the neuronal membrane are Arachidonic acid (C20:4, ω-6; ARA) and Docosahexaenoic Acid (C22:6, ω-3; DHA) which accumulate rapidly in the human brain during the first 1000 days, to support the fast increase in brain volume [144]. Docosahexaenoic Acid (C22:6, ω-3; DHA) and Arachidonic acid (C20:4, ω-6; ARA) contribute to the physical properties of membranes and have their own unique role in the development and function of the brain (Figure 24).
Adequate presence of especially Docosahexaenoic Acid (C22:6, ω-3; DHA) in the neuronal membranes is crucial, because it positively contributes to various processes important for neuronal growth and development, including modulating neural metabolism, differentiation, plasticity, neuroprotection and anti-inflammatory effects (see e.g. the excellent review of [145] on this topic). Arachidonic acid (C20:4, ω-6; ARA) is the precursor for specific membrane-derived eicosanoids that are important for immunity and immune responses, including the regulation of neuro-inflammation [146,147].
Other ω-3 LCPUFA in the brain, although present at a much lower concentration than DHA, include Eicosapentaenoic acid (EPA; C20: 5ω-3) and Docosapentaenoic acid (C22: 5, ω-3; DPA), which also release lipids mediators that play a role in the inflammatory response [145] and in particular EPA -but also DHA- which stimulates neurite growth during development [148].
DPA from the ω-6 family (C22: 5, ω-6; DPA) is the structural homologue of DHA and usually accumulates in the brain when the DHA supply is insufficient [149,150]. This compensation mechanism ensures that the total brain volume remains the same, although ω-6 DPA cannot fulfill the specific neurological roles of DHA [148,151,152]. High levels of ω-6 DPA are therefore considered disadvantageous.
Preferential accumulation of DHA in the brain occurs at high rate between the 3rd trimester up to 2 years and accumulation extends into childhood and adolescence [153,154]. This pattern runs parallel with active brain development and further illustrates the importance of DHA for brain development and function throughout life.
Brain lipids are not comparable to white adipose tissue (WAT) because a large part of the 'fat' in the brain is in the form of a myelin membrane which isolates the axons of neurons [155]. The myelin membrane is an extensive and highly specialized plasma membrane that is synthesized by myelinating glial cells: oligodendrocytes in the central nervous system (CNS) and Schwann cells in the peripheral nervous system (PNS) [156]
These specialized cells consist almost entirely of extracellular membrane and therefore have a high lipid content which gives the brain as a whole a high lipid concentration; moreover, these structural lipid compounds cannot be catabolized. The Fatty acid (FA) pattern differs inside the brain with different concentrations in the gray- and white- matter.
- The gray matter: localized to the gray-cortex with mainly brain cells, contains more saturated Fatty acids (FA) and Docosahexanenoic acid (C22:6, ω-3; DHA) compared to the white
- The white matter is located in the sub-cortical region and contains more Oleic acid (C18:1, ω-9; OA) and Docosatetraenoic acid (C22:4, ω-6; DTA) [74].
The Central Nervous System (CNS) is also unique because it does not use Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA), but only their desaturated and elongated Long-Chain PUFA (LCPUFA) derivatives [82]. PUFAs in the brain consist for over 90% of Docosahexaenoic acid ≈DHA (C22:6, ω-3; DHA) and Arachidonic acid (C20:4, ω-6; ARA) and the latter’s elongation product Docosatetraenoic acid (C22:4, ω-6; DTA) [157]. However, humans and other mammals are inefficient at synthesizing them from their respective 18-carbon precursors [45].
The biosynthetic process for very long chain fatty acids (VLCFA), PUFAs is complex and is rate [158,159].
For years, it was generally accepted that the biosynthesis of PUFAs takes place in the endoplasmic reticulum, which is also the most important site for phospholipid biosynthesis. It is believed that Docosahexaenoic acid (C22:6, ω-3; DHA), the major PUFA in adult mammalian brain and retina, is synthesized from dietary α-Linolenic acid (C18: 3, ω-3; ALA) in a route consisting of a series of elongase- and desaturation reactions. This route requires that ω-3 Docosapentaenoic acid (C22: 5, ω-3; DPA) is desaturated at position 4 by a microsomal acyl CoA-dependent Δ4 desaturase to form Docosahexaenoic acid (C22:6, ω-3; DHA). However, various studies have shown that such a Δ4 desaturase does not appear to exist. Instead, a 24-carbon ω-3 fatty acid was found to be synthesized, which is desaturated at position 6 to produce Tetracosahexaenoic acid (C24:6 ω-3; THA), followed by a round β- oxidation with Docosahexaenoic acid (C22:6 ω-3; DHA) as the end-product. Although still disputed, the peroxisome is the likely site of Tetracosahexaenoic acid (C24:6 ω-3; THA) β- oxidation. After its formation, Docosahexaenoic acid (C22:6 ω-3; DHA) is returned to the endoplasmic reticulum, where it is esterified into membrane lipids. The Sprecher’s shunt is depicted in detail in Figure 25, with its revised route for the biosynthesis of Docosahexaenoic acid (C22:6 ω-3; DHA). The synthesis of Arachidonic acid (C20: 4 ω-6; ARA) and Docosapentaenoic acid (C22: ω-3; DPA) from dietary Linoleic acid (C18:2, ω-6; LA) in the feed follows a similar path [61,78]. The Peroxisomal Pathways for VLCFAs are called ‘Sprecher's shunt’ [61-66] (Figure 8 & 25).
Peroxisomal Pathways for VLCFA (‘Sprecher's shunt’ elucidated in detail)Fatty acid (FA) metabolism occurs in both mitochondria and peroxisomes with some differences, short and medium chain length FA being oxidized in mitochondria, whereas very long chain fatty acids (VLCFA) are metabolized in peroxisomes, after which the metabolites (shorter in chain length) are transported into mitochondria for subsequent oxidation to CO2 and H2O. VLCFAs enter peroxisomes as CoA-esters via specific half-ABC transporters [160] but they cannot enter mitochondria. Medium and long chain length FA pass across the mitochondrial membrane as a complex with carnitine.
In humans, Docosahexaenoic acid (C22:6, ω-3, DHA) is either obtained from the diet or may be converted in small amounts from Eicosapentaenoic acid (EPA, C20:5, ω-3) via Docosapentaenoic acid (DPA, C22:5 ω-3). This synthesis was long thought to occur through an elongation step (C20/22) followed by the action of Δ4-desaturase. It is now considered more likely that Docosahexaenoic acid (C22:6, ω-3, DHA) is biosynthesized via a C24 intermediate followed by β-oxidation in peroxisomes. Thus, Eicosapentaenoic acid (EPA, 20:5, ω-3) is twice elongated, yielding Tetracosapentaenoic acid (C24:5 ω-3; TPA), then desaturated to Tetracosahexaenoic acid (C24:6 ω-3; THA), then shortened to DHA (22:6 ω-
3) via β-oxidation. This pathway is known as Sprecher’s shunt [61-66].
Peroxisomal enzymes also play a role in Docosahexaenoic acid (C22:6, ω-3, DHA) formation from the precursor Tetrasahexaenoic acid (C24:6, ω-3; THA) [61]. Docosahexaenoic acid (C22:6, ω-3; DHA), the major FA of the ω-3 series, has different biological actions such as modulation of cell functions and metabolic effects on lipid metabolism [76,161]. In humans, DHA levels depend largely on dietary intake, but it can also be synthesized, albeit not very efficiently [162] from the precursor, the Essential Fatty acid (EFA) α-Linolenic acid (C18:3, ω-3; ALA). ALA is converted, via elongation and desaturation reactions in microsomes, to Tetrasahexaenoic acid (C24:6, ω-3; THA) which is then β-oxidized in peroxisomes to give the final product Docosahexaenoic acid (C22:6, ω-3, DHA) (Figure 25) [163]. Latest developments related to ‘Sprecher’s shunt’ concern the research of [66], where in a rat model it was demonstrated in vivo for the first time that Docosahexaenoic acid (C22:6, ω-3, DHA) is both a product and a precursor of Tetracosahexaenoic acid (C24:6 ω-3; THA) (Figure 25; comparison AD 1991 vs 2018).
Figure 25: ω-3 PUFA biosynthesis pathway determined in 1991, compared to the state of affairs in 2018. [1] D6-desaturation, elongation and D5- desaturation converting ALA to EPA, [2] elongation of EPA to DPAn-3, [3] elongation of DPAn-3 to TPAn-3, [4] D6-desaturation of TPAn-3 to THA, [5] previously discovered peroxisomal b-oxidation of THA to DHA in 1991, [6] retroconversion of DHA to EPA, [7] theoretical D4-desaturation of DPAn-3 to DHA reported in humans and [8] newly reported elongation of DHA to THA in the present 2018 study. ALA – a-linolenic acid, 18:3n-3; DPAn-3 – docosapentaenoic acid, 22:5n-3; TPAn-3 – tetracosapentaenoic acid, 24:5n-3; THA – tetracosahexaenoic acid, 24:6n-3
Docosahexaenoic acid (C22:6 ω-3; DHA) has played a unique role during evolution of the modern hominid brain [20] and adequate intakes of ω-3 polyunsaturated fatty acids (PUFA), especially of Docosahexaenoic acid (C22:6 ω-3; DHA), are very important throughout life, especially in the perinatal period, when it is supplied by the mother to the foetus through the placenta, and then to the new-born through lactation. Maintenance of adequate DHA intake into old age is crucial to prevent the onset of brain dysfunctions [59]. A continuous supply of FAs to the CNS and their replacement in CNS membranes take place throughout life, a major role being played by the liver in the processes involving FA metabolism and delivery to peripheral organs, including the brain [164]. In addition, the production of anti-inflammatory and protective compounds (resolvins and neuroprotectins) derived from Docosahexaenoic acid (C22:6 ω-3; DHA) has been described (Figure 20; [165].
Very interestingly, a metabolic pathway similar to that of Arachidonic acid (C20:4, ω-6; ARA) has also been described for Linoleic acid (C18:2, ω-6, LA), the essential fatty acid (EFA) of the ω-6 series, with the first steps taking place in microsomes and the last step of β- oxidation in peroxisomes. In more detail, when cells were incubated with relatively high concentrations of Arachidonic acid (C20:4, ω-6; ARA) the formation of 24 carbon FA with 4 and 5 (desaturated in position 6) double bonds was activated, suggesting an inducible, concentration-dependent (peroxisomal) metabolic pathway for the formation of Osbond Acid (C22:5, ω-6; OA) [166,167].
Conjugated linoleic acid (CLA) is also catabolized in peroxisomes. The term Conjugated Linoleic acid (CLA) refers to a mixture of positional and geometric isomers of Linoleic acid (C18:2, ω-6; LA) characterized by conjugated double bonds not separated by a methylene group as in Linoleic acid. These double bonds are located at position 8 and 10, 9 and 11, 10 and 12, 11 and 13 in cis or trans configuration. CLA is naturally produced in the rumen, for example of the cow, by bio-hydrogenation, and thus it is present in milk, dairy products, and meat. In this case, the predominant isomer is the cis-9, trans-11 also known as Rumenic acid (C18:2, ω-7; RA) [168].
Benefits of EPA
The ultimate goal of using ω-3 fatty acids is the reduction of cellular inflammation, since eicosanoids, derived from Arachidonic acid (C20:4, ω-6; ARA), an omega-6 fatty acid, are the primary mediators of cellular inflammation (Figure 20). Eicosapentaenoic acid (20:5, ω-3; EPA) is the most important of the ω-3 fatty acids to reduce cellular inflammation for a number of reasons. First, Eicosapentaenoic acid (C20:5, ω-3; EPA) is an inhibitor of the enzyme ∆5-desaturase (D5D) that produces Arachidonic acid (C20:4, ω-6; ARA). The more Eicosapentaenoic acid (C20:5, ω-3; EPA) is present in the diet, the less Arachidonic acid (C20:4, ω-6; ARA), is produced [170]. This essentially chokes off the supply of Arachidonic acid (C20:4, ω-6; ARA), needed for the production of pro-inflammatory eicosanoids (prostaglandins, thromboxanes, leukotrienes, etcetera Figure 26).
Docosahexaenoic acid (C22:6, ω-3; DHA) is not an inhibitor of ∆5-desaturase (D5D) because it does not fit into the active catalytic site of the enzyme due to its larger spatial size. Secondly, as an additional safety policy, Eicosapentaenoic acid (C20:5, ω-3; EPA) also competes with Arachidonic acid (C20:4, ω-6; ARA) for the enzyme phospholipase A2 necessary to release Arachidonic acid (C20:4, ω-6; ARA) from the membrane phospholipids (where it is stored). Inhibition of this enzyme is the mechanism of action used by corticosteroids [170]. When adequate levels of Eicosapentaenoic acid (C20:5, ω-3; EPA) are ascertained to compete with Arachidonic acid (C20:4, ω-6; ARA) (i.e. a low AA/EPA ratio), one can profit from many of the benefits of corticosteroids but without their side effects. This is due to the fact that Arachidonic acid (C20:4, ω-6; ARA) is not released from the cell membrane preventing the production of inflammatory eicosanoids. Because of its increased spatial dimensions, Docosahexaenoic acid (C22: ω-3; DHA) is not a good competitor of phospholipase A2 in comparison with Eicosapentaenoic acid (20:5n-3; EPA). On the other hand, Eicosapentaenoic acid (C20:5, ω-3; EPA) and Arachidonic acid (C20:4, ω-6; ARA) are very similar spatially so they are in constant competition for the phospholipase A2 enzyme just as both fatty acids are in constant competition for the ∆5- desaturase enzyme. This is why measuring the AA/EPA ratio is such a powerful predictor of the state of cellular inflammation in organs like e.g. brain. The only way to control cellular inflammation in the brain is to maintain high levels of Eicosapentaenoic acid (C20:5, ω-3; EPA) in the blood [172].
EPA in the human brain
Finally, it is often assumed that absence of high levels of Eicosapentaenoic acid (20:5n-3; EPA) in the brain, implies that it is not important for neurological function [17]. Actually, it is key for reducing neuro- inflammation by competing with Arachidonic acid (C20:4, ω-6; ARA) for access to the same enzymes needed to produce inflammatory eicosanoids. However, once Eicosapentaenoic acid (20:5n-3; EPA) enters the brain, it is rapidly oxidized [173,174]. This is not the case with DHA [175]. The only way to control cellular inflammation in the brain is therefore to maintain high levels of Eicosapentaenoic acid (20:5n-3; EPA) in the blood [172]. This is why all research on depression, ADHD, brain trauma, etcetera have demonstrated Eicosapentaenoic acid (20:5n- 3; EPA) to be superior to Docosahexaenoic acid (C22: ω-3; DHA) [176].
Benefits of DHA
Eicosapentaenoic acid (20:5n-3; EPA) and Docosahexaenoic acid (C22: ω-3; DHA) are the most important PUFAs in the body. The role and function(s) of the omega-3 EPA is extensively discussed while that of DHA is often neglected because it is not understood. In addition, Docosahexaenoic acid (C22: ω-3; DHA) is hardly measured in the human brain [15] which means that the equally important function of this other ω-3 PUFA is underexposed.
The first difference is in the area of ω-6 fatty acid metabolism. Whereas Eicosapentaenoic acid (20:5n-3; EPA) is the inhibitor of the enzyme ∆5-desaturase (D5D) that directly produces Arachidonic acid (C20:4, ω-6; ARA), Docosahexaenoic acid (C22: ω-3; DHA) is an inhibitor of another key enzyme ∆6-desaturase (D6D) that produces the first metabolite from Linoleic acid (C18:2, ω-6; LA) known as γ-Linolenic acid (C18:3, ω-6; GLA) [177]. However, this is not exactly an advantage. Even though reduction of γ-Linolenic acid (C18:3, ω-6; GLA) will eventually decrease Arachidonic acid (C20:4, ω-6; AA) production, it also has the more immediate effect of reducing the production of the next metabolite known as Dihomo-γ-Linolenic acid (C20:3, ω-6; DGLA). This may have disastrous effects as a great number of powerful anti-inflammatory eicosanoids are derived from Dihomo-γ- Linolenic acid (C20:3, ω-6; DGLA). This is why the use high-dose Docosahexaenoic acid (C22: ω-3; DHA) must essentially be combined with adding back trace amounts of γ- Linolenic acid (C18:3, ω-6; GLA) in order to maintain sufficient levels of Dihomo-γ- Linolenic acid (C20:3, ω-6; DGLA) to continue the production of anti-inflammatory eicosanoids [82].
It must be acknowledged that the key benefit of Docosahexaenoic acid (C22: ω-3; DHA) lies in its unique spatial characteristics. As mentioned earlier, the extra double bond (6 in DHA versus 5 in EPA) and increased carbon length (22 carbons in DHA vs. 20 in EPA) means that DHA takes up takes up a lot more space than does EPA in the membrane. Although this increase in spatial volume makes DHA a poor substrate for phospholipase A2 as well as the COX and LOX enzymes, it has the advantage of making membranes (especially those in the brain) a lot more fluid as the DHA sweeps out a much greater volume in the membrane than does EPA. This increase in membrane fluidity is critical for synaptic vesicles and the retina of the eye as it allows receptors to rotate more effectively thus increasing the transmission of signals from the surface of the membrane to the interior of the nerve cells. This is why DHA is a critical component of these highly fluid portions of the nerves [178]. On the other hand, the myelin membrane is essentially an insulator so that relatively little DHA is found in that type of membrane [179].
As mentioned in our previous review [9], evolutionary sciences studying the problem of human brain growth (encephalization) can trace their foundations back to embryology and fetal development of the brain during gestation and is therefore important because they are an eventual equal representation of the early cerebral development of the first hominids. In fact, they indicate exactly which building materials were needed during the evolution of the human brain with its tremendous expansion from Homo erectus (≈950 cm3 brain), towards modern Homo sapiens (≈ 1500 cm3 brain) [54].
It is important to note that once the ω-3 PUFA levels are established in the course of evolutionary brain development, these changes cannot be fully repaired by supplementing with ω-3 LCPUFA during later life stages [180-182], Higher levels of ω-3 LCPUFA are associated with better motor performance [183], higher scores in cognitive functioning [184,185] and better neurological scores [186. In addition, low DHA and ω-3 LCPUFA or increased ω-6 LCPUFA concentration in adult brain is, from an evolutionary perspective, related to a negative selection pressure such as neuropsychiatric disorders like depression [187-189]. Although depression from an evolutionary point of view implies a higher form of consciousness as a complex form of social hierarchy [190], it is an undesired trait. These studies show that DHA in the brain is crucial for brain function and mental health in humans, and further underlines the importance of adequate DHA accumulation until an age of approximately 7 years. Brain development in humans -also called ‘synaptic pruning’- is influenced by food for up to 7 years old and then stops [191]. Such specific nutritional conditions could possibly have been provided during early Paleolithic times due to eating at a regular base ruminant meat (Syncerus caffer) during the early years of life especially until an age of approximately 7 years old [183-186].
Common Effects for both EPA and DHA
Not surprisingly, there are some areas in which both EPA and DHA appear to be equally beneficial. As an example, both are equally effective in reducing triacylglycerol levels [192]. This is probably due to the relatively equivalent activation of the gene transcription factor (PPAR-α) that causes the enhanced synthesis of the enzymes that oxidize fats in lipoprotein particles. There is also apparently equal activation of the anti-inflammatory gene transcription factor PPAR-γ [193]. Both seem to be equally effective in making powerful anti-inflammatory eicosanoids known as resolvins [165]. Finally, although both have no effect on total cholesterol levels, DHA can increase the size of LDL particles to a greater extent than can EPA [192]. In general, PUFAs, especially ω-3 fatty acids such as eicosapentaenoic acid (EPA; C20:5 ω-3) and docosahexaenoic acid (DHA; C22:6 ω-3) have been documented to have significant influence on biochemical and physiological changes in the body and to exert positive influences on human nutrition and health [194]. For a clear definition for all physiological and biochemical processes involved, one is referred to the review of [195].
Conclusions and Perspectives
The Savanna Dry Land (SDL) hypothesis assumes that the African savannah supplied all nutritional elements and other chemical compounds to explain the uniqueness of the human brain with its overgrown neocortex [28]. Over against this scientific paradigm [29] we have the 60-year-old 'Aquatic Phase Hypothesis' (APH) or Hardy / Morgan hypothesis [30] who states that our ancestors must have had an aquatic water phase with aquatic (sea) food with essential omega- 3 fatty acids to explain this enormous brain growth of the human brain [24,31,32]. The followers of the APH evolution model have challenged SDL supporters to present a biochemical model explaining the development of large human brains [32].
In this review, we have provided such a biochemical model based on LC-MS measurements of a juvenile High Fat (HF)-diet, obese C57bl6 mouse model, raised on bovine fat -mainly unsaturated fatty acids with very long chains (VLC-FA; C48-C56 Triacylglycerols, (TGs)) - that accumulate throughout the brain [12]. The described HF-diet with 24.0% bovine lard caused a very significant increase of Lyso-phosphatidylcholine, a preferred carrier, as a by-product of PUFAs through the blood-brain barrier [197]. This study with rodent models indicaties that Fatty Acids (FAs) can pass the Blood Brain Barrier (BBB) [194]. These important observations were made by happenstance and possibly indicate the role of bovine lard in human encephalization during the course of evolution. Furthermore, in post-mortem brains from human donors, we found that Triacylglycerols (TGs) are the most important lipid fraction in human brain neocortex (≈68,32% white versus ≈75,96% gray matter), suggesting the importance of this lipid fraction in brain encephalization [195]. We also described a clear correlation between hunter and prey based on similarities in migration patterns of the African buffalo (Syncerus caffer) and those of early hominids [14]. Our results show that large ruminants from the African savannah probably played a highly important role in the exponential brain growth from early hominids to the modern Homo sapiens.
Here we present the 'African Buffalo Savannah hypothesis' ('ABS hypothesis') which provides strong evidence that the meat and bovine lard of herds of the early African buffalo on the African savannah in the Pleistocene had such a specific biochemical composition that it could have been the evolutionary driving force behind the encephalization of the early hominids. Another finding that supports the 'ABS hypothesis' is the previously published map of migration routes of early African cattle (ancestors of Syncerus caffer) and early hominids - supporting the hunter versus prey correlation as an important element of the 'ABS hypothesis' [14].
So, it is imperative to explain in our ‘ABS’ biochemical model how the two important ω3 ‘fish-oils’ PUFAs EPA (C20:5, ω-3) and DHA (C22:6, ω-3) are produced by the African buffalo. And in addition, how significant amounts of TGs are produced for the developing human brain.
The African buffalo forages on the African savannah and has access to seed material from both grasses such as African bromegrass grass (Bronus biebersteinii) and higher savannah vegetation. The most important fatty acid of grass meadow is α-linolenic acid (C18: 3, ω3; ALA) [200], while the seed material is usually a rich source of linoleic acid (C18: 2, ω6; LA) [201]. Isomeration reactions due to microbiome in the rumen convert LA into its isomer, cis-9, trans-11 conjugated linoleic acid (CLA) which in turn is reduced to vaccenic (VA) fatty acid [202]. In the next stage, VA is converted to stearic fatty acid (C18:0). For biohydrogenation of LNA, a similar pattern of isomeration reactions is observed followed by a sequence of reductions and concluded by the formation of stearic fatty acid (C18:0). In tissues, Δ9-desaturate converts the stearic fatty acid into C18:1 (Oleic Acid) [65]. The C:18 MUFAs are required as a FA ‘tail’ for the glycerol C-backbone in order to produce VLCFAs (TGs) [203]. After leaving the rumen, the free fatty acids (FFAs) from the food and bacteria particles are desorbed by lysolecithin and bile salts. The micelles are formed and taken up by the epithelial cells of the jejunum. The FFAs are released from the small amounts of triacylglycerols and glycolipids that reach the intestines [65]. The metabolism of fatty acids in the human body is then started. However, despite the described conversions of the two EFAs in the rumen, ruminants may have the ability to dehydrogenate and extend linoleic acid (C18: 2, ω-6; LA) to Docosahexaenoic acid (C22: 6, 1-3; DHA) [65]. The main PUFA in the meat of Syncerus caffer is Docosapentaenoic acid (C22: 5, ω3; DPA [16]. Conversion of Docosapentaenoic acid (C22: 5, ω-3, DPA) to the important brain ‘fish oil’ DHA (22: 6, ω-3) was earlier suggested to occur by ∆4-desaturation activity in these species [200]. However, this ∆4-desaturation model has been replaced by another biochemical model with a role for peroxisomes, the pathway nowadays known as the "Sprecher’s shunt" [61-66]. So, the biohydrogenation of Docosahexaenoic acid (C22: 6, 3-3; DHA) from Eicosapentaenoic acid (C20: 5, ω-3; EPA) follows the shunt of Sprecher in the peroxisome [61-67].
These PUFA conversion routes in the tissues of the African buffalo and its relation to feed, need to be confirmed by robust LCMS measurements. An outline of targeted experiments related to this topic is given in Table 5.
Dietary fatty acids are used as building blocks for the developing brain
Table 5: Experimental program for a first 2 years scientific research program demystifying the evolutionary forces of the mysterious mammalian brain based on a C57bl6 mouse model with ‘overgrown brain’
|
No. |
Action points & Scientific activity and targeted experiments |
Duration |
|
1 |
Repeat brain steatosis experiment in a C57bl6 mouse model (n=20) raised on 24% bovine lard individually housed to prevent social hierarchy (12. van Ginneken et al 2017). |
6 months |
|
2 |
Because rats are more intelligent similar brain steatosis experiment in rats (n=20) individually housed raised on 24% bovine lard |
6 months |
|
3 |
Collect neocortex material and plasma from donors (NBB) of stillborn infants (n=10) vs. 30 years age (n=10) |
6 months |
|
4 |
LCMS measurements feed, overgrown C57bl6 mouse- & rat-model neocortex material & plasma of NBB see no.3 (12. van Ginneken et al 2017). |
6 months |
|
5 |
Explain general exemplified topics of neurophysiology like neural networks, frequency codes, synaptic characteristics, neurotransmitters (201. van Ginneken et al 1995) |
2 years |
|
6 |
Continue 24% bovine lard nutritional/breeding program of C57bl6 mouse model (10 generations) for testing fixation of expression patterns in the genes by epigenesis |
2 years |
|
7 |
Similar continuation of 24% bovine lard nutritional/breeding program of rat model (5 generations) for testing fixation of expression patterns in the genes |
2 years |
|
8 |
Perform maze test rats (5 generations) with overgrown brains (202. Service 1994). |
2 years |
|
9 |
Collect in Africa: African buffalo (Syncerus caffer) (16. Crawford et al 1969) and African savannah grass & seeds (200. Benbrook et al 2018; 201. Chilliard et al 2001) and perform LCMS at meat, lard, paunch. |
2 years |
|
10 |
Genetic model of ‘brain steatosis’ will be fixed in the genes of mouse (10 generations) rats (5 generations), whole genome sequencing (207. Foley et al 2018). |
2 years |
|
11 |
Increased brain volume towards Computational & Mathematics model |
2 years |
The developing brain of early hominids at the African savannah relies to a great extent on the Long Chain Poly Unsaturated Fatty Acid (LCPUFA) plasma pool for Docosahexaenoic acid (C22:6, ω-3; DHA) accumulation. Although the brain can generate some Docosahexaenoic acid (C22:6, ω-3; DHA) by endogenous synthesis of precursor ω-3 LCPUFA in glial cells [208], Docosahexaenoic acid (C22:6, ω-3; DHA) synthesis in the brain takes place at a much lower rate than the total rate of brain growth from DHA needed to support growth and development [209]. In plasma, Docosahexaenoic acid (C22:6, ω-3; DHA) and other LCPUFA are present in two large pools: A). Bound albumin, as non-esterified fatty acid (NEFA) -DHA or lyso-phosphatidylcholine- esterified (LPC) -DHA [197]; B) Esterified in lipoproteins to e.g. TG, PL, cholesterol, PL or other lysophospholipids. There is no consensus as to which lipid form in the plasma pool is preferably used for accumulation in the brain [211-213].
, nor have the mechanisms been agreed by which these different forms of circulating PUFA are delivered - either by protein-mediated and / or passive diffusion [214]. The plasma to tissue uptake process appears to be non-selective for ω-6 and ω-3 LCPUFA. The accumulation of Docosahexaenoic acid (C22:6, ω-3; DHA) in the brain is therefore dependent on the total and relative levels of Docosahexaenoic acid (C22:6, ω-3; DHA) and other ω-3 and ω-6 LCPUFA in the plasma [215,216]. The capacity of LCPUFA synthesis from Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA) is low in humans [217,218]. However, Docosahexaenoic acid (C22:6, ω-3; DHA) accumulation in the developing brain of early hominids is not only dependent on the supply of preformed Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA) from African savannah nutritional resources like meat and lard of Syncerus caffer. The biosynthesis of LCPUFA from Linoleic acid (C18:2, ω-6; LA) and α- Linolenic acid (C18:3, ω-3; ALA) in the liver and other tissues involves a series of steps of extension and desaturation as extensively described earlier in this review. The same set of enzymes is used for the conversion of Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA) to Arachidonic acid (C20:4, ω-6; ARA) and Docosahexaenoic acid (C22:6, ω-3; DHA) and therefore compete with each other. For example, high nutritional supply of Linoleic acid (C18:2, ω-6; LA) inhibits endogenous Docosahexaenoic acid (C22:6, ω-3; DHA) synthesis and results in higher ω-6 LCPUFA in the circulatory system, which further limits the uptake of Docosahexaenoic acid (C22:6, ω-3; DHA) in the developing brain, because circulating ω-3 and ω-6 LCPUFA also compete for uptake by the brain [219,220]. Very interestingly, in a piglet study it was found that a formula with a high Linoleic acid (C18:2, ω-6; LA) increased the accumulation of ω-6 LCPUFA and reduced Linoleic acid (C18:2, ω-6; LA), α-Linolenic acid (C18:3, ω-3; ALA) and other ω-3 LCPUFA in the brain. In addition, the increasing Docosahexaenoic acid (C22:6, ω-3; DHA) content was not able to prevent some of the disorders in the fatty acid profile of the brain caused by the high supply of Linoleic acid (C18:2, ω-6; LA) [151].
Having gained a comprehensive understanding of the literature related to LCPUFA conversions and brain delivery, in Part 3 of this three-part series entitled: [Review] Where Darwin neglected to explain the human-brain encephalization: Part 3: “Explore the mysteries of mysteries, the unexplained evolutionary mechanism behind the overgrown human brain following a lipidomics based approach”, I will present LCPUFA conversion measurements in human brains from 16 donors of the Netherlands Brain Bank (Amsterdam) supported by observations at a C57bl6 mouse model (whole brain).
Yet, in this review (part 2), the central theme is the "Savannah Dry Land Hypothesis" (SDLH) which assumes that the African savannah could have supplied all nutritional elements and other chemical compounds to explain the uniqueness of the human brain with its overgrown neocortex [28].
Central to the SDLH is that the cradle of modern humanity stood in East Africa - a country with often harsh and unsTable environments and resources, and with enormous evolutionary selection pressure for favorable properties. Early hominids, in the transition from Homo habilis to Homo erectus for more than 200,000 years, may perhaps have overcome these severe conditions by, among other things, improved tools to hunt for more available meat from the most abundant herbivore and ruminant of the African savannah, the African buffalo Syncerus caffer (or its ancestor) for which a total biomass of 54.6 ± 10.9 kg km-2 has recently been recorded [49]. In addition, we have demonstrated in a C57bl6 mouse model fed on a High fat diet based on 24% bovine lard and 0.5% cholesterol the occurrence of "brain steatosis" of very long chain polyunsaturated fatty acids (VLCFAs) supplied by the bovine lard [12]. Never before has brain expansion through nutritional intervention been observed in a mouse model with regard to the accumulation of TGs as a result of a fat-based diet on lard. Only studies with domestic cats - giving a meat diet for six months [47] were comparable to our rodent study and these also indicated VLCFA accumulation in the brain supporting our first hypothesis. So, our C57bl6 mouse model, with its short reproduction period, is a new and attractive "tool" that offers the evolutionary biologist unprecedented and unlimited possibilities to test the various physiological, biochemical and genetic evolution processes associated with encephalization in a mammal in the laboratory.
In the remainder of this review, we will argue that our ecological "Savannah Dry Land Hypothesis (SDL-hypothesis)" - based on a powerful description of the many enzymatic metabolic VLCPUFA conversions - provides strong evidence that the meat and lard African buffalo (Syncerus caffer), hunted by early hominids on the Pleistocene's African savannah had such a specific biochemical composition that it could have been the evolutionary driver for encephalization of the early hominids [14]. Proponents of the evolutionary “Aquatic Phase Model (APM)”' claim that the endogenous synthesis of Docosahexaenoic acid (C22: 6, ω-3; DHA) from α-Linolenic acid (C18: 3, ω-3; ALA) in humans is much lower and more limited than previously assumed due to competing enzymatic systems [209]. Rate limiting steps in enzymatic conversions due to 'delayed' enzymatic reactions are the ∆6-desaturase steps for both essential fatty acids (EFAs), Linoleic acid (C18: 2, ω-6, LA) and α-Linolenic acid (C18: 3, ω -3; ALA), and the ∆5-desaturase step converting Dihomo-ℽ-Linolenic acid (C20: 3, ω-6; DGLA) into Arachidonic acid (C20: 4, ω-6; ARA). In the previous sections of this review, we have described very exciting discoveries and interconversions in different desaturase / elongase routes of FAs which sustain the SDL-hypothesis.
Syncerus caffer grazes during the wet season, but increases its changing behavior in the dry season, wherever it is found in forest habitats. Andropogon gayanus turned out to be the most preferred grass species [10]. TUFA content was composed of P2O, P2L, PO2, POL, O3, PL2, O2L, L2O and L3 with P = palmitic acid (C16: 0) and L = linoleic acid (C18: 2, ω-6; LA) [70]. Two important conclusions can be drawn: First, all combinations can be found on the glycerol backbone molecule. Second, the most important EFA is an inflammatory an ω-6, linoleic acid (C18: 2, ω-6; LA). These findings affect the meat and the biochemical composition of Syncerus caffer as a prey for early hunting of hominids. It is noteworthy that PUFAs of the ω-6 series occur more often in the tissues of the African buffalo than those of the ω-3 series. Because the C18 and C20 acids mainly come from the ω-6 series, it was to be expected that the C22 acids would also have the same parental origin.
We are interested in the fatty acid composition of the meat of the African buffalo (Syncerus caffer) because it could have played a role in encephalization of modern archaic man Homo sapiens who originated on the African savannah. We propose that ruminants can convert low digestible savannah grass into high-quality meat and lard, important for early brain hominid growth. Mammals lack Δ12 and Δ15-desaturase activities [45], so they cannot synthesize Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA) from the precursor Oleic acid (18:1, ω-9; OA) [97].
In addition, it is important to uncover the fate of these two Essential Fatty acids (EFAs), LA and ALA, in the rumen. Via isomerization and reduction reactions, the ω-3 EFA α-Linolenic acid (C18:3, ω-3; ALA) is converted via Rumenic acid (C18:2, ω-7; RA) and Vaccenic acid (C18:1, ω-7; VA) into Stearic acid (C18:0; ω-9; SA). In addition, isomerization of the ω-6 EFA Linoleic acid (C18:2, ω-6; LA) leads to the formation of Rumenic acid (C18:2, ω-7; RA) which in turn is also converted via reductase reactions into Stearic acid (C18:0; ω-9; SA) [65].
It is assumed -but presently not yet investigated- that all ∆4, ∆5, ∆6, ∆8, ∆9, ∆12, ∆15, ∆17 desaturase enzymatic reactions in conjunction with a ∆5, ∆6, ∆9 elongase enzymatic activity take place in the paunch of a ruminant like the African buffalo Syncerus caffer. This assumption is based on similar findings on bio-synthetic pathways of polyunsaturated fatty acids (PUFAs) in microalgae [85].
In addition, ∆8-desaturase activity might be ‘a new solution’ to produce EPA, so important for the human brain through the route by which the inflammatory Eicosadienoic acid (C20: 2, ω-6; EDA) is converted into Dihomo-γ-Linolenic acid (C20: 3, ω-6; DGLA) via ∆8- desaturase and through the non - inflammatory Eicosatrienoic acid (C20: 3, ω-3; ETrA) from which by ∆8 desaturase activity the non-inflammatory product Eicosatetraenoic acid (C20: 4, ω-3; ETA) could be produced [78]. Dihomo-γ-Linolenic acid (C20: 3, ω-6; DGLA) is the direct precursor of prostaglandin E1 and thromboxane B1. Dihomo-γ-Linolenic acid (C20: 3, ω-6; DGLA) and Eicosatetraenoic acid (C20: 4, ω-3; ETA) are also direct precursors of the long chain polyunsaturated fatty acids (LC-PUFAs) Arachidonic acid (C20: 4, ω-6; ARA) and the for human brain so important Eicosapentaenoic acid (C20: 5, ω-3; EPA) respectively. Finally, after leaving the rumen, the FFAs from the particles of food and bacteria are desorbed by lysolecithin and bile salts. The micelles are formed and taken up by the epithelial cells of the jejunum. The FFAs are released from the small amounts of triacylglycerols and glycolipids reaching the intestines [65]. Subsequently, lipids are converted to meat and lard of Syncerus caffer.
Digestion of triacylglycerols absorbed from the diet can occur by several routes through their Fatty Acyl CoAs: (a). β-oxidation to provide energy in the form of ATP; (b). esterification in cellular lipids including triacylglycerols, cholesteryl esters (ChE) and phospholipids and (c). converted into their important longer chain and more unsaturated products derived from a series of desaturation and elongation reactions that mainly take place in the liver and to a lesser extent in other tissues [98]. Dietary fat is mainly taken up in the form of triacylglycerols containing the fatty acids Palmitic acid (C16: 0, ω-7; PA) and Oleic acid (C18: 1, ω-9; OA) [130]. As mentioned earlier, mammals lack activities of ∆12 and ∆15 desaturase, so they cannot synthesize Linoleic acid (C18: 2, ω-6; LA) and α-Linolenic acid (C18: 3, ω-3; ALA) from the precursor Oleic acid (18: 1, ω-9; OA). With regard to "Sprecher’s shunt", the meat of the African buffalo (Syncerus caffer) has such a specific biochemical composition through this biochemical ‘shunt’ that it could have provided conjugated linoleic acid (CLA) to the early hunting hominids at the African savannah.
Enzymatic conversion takes place via the enzymatic path of a ∆6-desaturase in the PUFA scheme (which is normally the rate limiting step towards Arachidonic acid (C20: 4, ω-6; ARA)), while a new enzymatic route proceeds via γ-linolenic acid (C18: 3, ω-6; GLA) and via a C18 / 20 elongase towards Dihomo- γ-linolenic acid (C20: 3, ω-6; DGLA), and subsequently via ∆5 desaturase to Arachidonic acid (C20: 4, ω-6; ARA). This is a very specific pathway and is characteristic for ruminants [92].
Syncerus caffer has exceptionally high amounts of important EFAs: about 17.3% for Linoleic acid (C18: 2, ω-6; LA), and about 5.4% for α-Linolenic acid (C18: 3, ω-3; ALA) and about 7.5% for Arachidonic acid (C20: 4, ω 6, ARA). Of the C18 fatty acids (EFAs) in the elongase/ desaturase array, Linoleic acid (C18: 2, ω6; LA) is most common in muscle tissue. Based on product / precursor ratios we determined possible ∆17-desaturase, ∆8-desaturase and ∆4-desaturase enzymatic activity as a consequence of microbiome activity in the rumen. Also typical and characteristic for this ruminant is ‘Sprecher’s shunt’, which might underlie the typically observed meat composition of Syncerus caffer with extremely its high concentrations of Arachidonic acid (C20: 4, ω-6; ARA) and Docosapentaenoic acid (C22: 5, ω-3; DPA) [20]. It has also been suggested that Docosapentaenoic acid (C22: 5,;-3; DPA) -abundant in meat- may have functioned as a buffer between the two major ω-3 PUFAs Eicosapentaenoic acid (C20: 5, ω-3; EPA) and Docosahexaeinoic acid (C22: 6) , ω-3; DHA) for early hominid brains [75].
Digestion and absorption of fats in the diet, including those in beef, depend to some extent on the size and degree of unsaturation of the fatty acids and on the structure of the fat molecules which implies the position of different fatty acids at the glycerol backbone in molecules of TAGs. Digestion of fats in humans is accomplished by lipase enzymes that hydrolyse the ester bonds, releasing the fatty acids from glycerol. In the small intestine ‘pancreatic lipase’ removes the #1 and any remaining #3 fatty acids, leaving a monoacylglycerol with only the #2 fatty acid [119]. Short-chain fatty acids can be absorbed directly from the intestine and transported to the liver. Long-chain saturated fatty acids (>18 C) tend to form insoluble hydrated acid soaps in the less acidic environment of the small intestine, particularly when calcium and magnesium are present [57]. Absorption of fatty acids depends on their solubility (which is greater for unsaturated fatty acids and shorter chain saturated fatty acids) and their position on the glycerol backbone. For example, in beef fat, Palmitic acid (C16: 0; ω-7; PA) is in sn-1 position and Oleic acid (C18: 1; ω-9; OA) is in sn-2 position as seen in POO, POP, and POS TAGs but varies in the fatty acids that are in sn- 3 (with P: Palmitic acid; O: Oleic acid; S: Stearic acid). ‘Lard’ has a very characteristic composition of each main fatty acid in the sn-2 position of the triacylglycerol in comparison to other animal fats (butter & tallow), with Palmitic acid (C16:0, ω-7; PA) at the sn-2 position in every combination (1 or 2 or 3) [118,124]. In a recent study with ten-week-old Golden Syrian male hamsters, [221] confirmed the hypothesis that the incorporation of docosahexaenoic acid (DHA) in tissues will be higher when it is ingested as triacylglycerols (TAG) structured at the sn-2 position, which enhances efficacy and health benefits of dietary DHA ω-3 supplementation [221]. This is an important observation indicating that to a great extent the biochemical composition of the human brain is mainly provided by the ω-3 PUFA Docosahexaenoic Acid (C22:6, ω-3; DHA).
Providing a more optimal balance of ω-3 and ω-6 PUFA during early brain development can be instrumental. This may have been achieved in early hominids with developing brains at the African savannah by a balanced Paleolithic diet with a recommended ω-6/ω-3 ratio of around 1/1≈one [222].
The human brain consists of about 75 percent water, while it is the fattest organ in our body. Around 60% of the structural material of the brain (dry weight) is lipid. The brain contains about 100 billion brain cells interrelated by axons and dendrite conducting electrical impulses coding for information from or to the periphery. Phospholipids (PL) are phosphodiesters (PDE) linked to a base, in the brain usually ethanolamine, as phosphatidylethanolamine (PE). PE binds two different types of Fatty acids (FA), namely unsaturated Palmitic acid (C16:0, ω- 7; PA) and Stearic acid (C18:0; ω-9; SA), as well as Polyunsaturated Fatty acids (PUFAs) like Arachidonic acid (C20:4,-ω-6; AA) and Docosahexaenoic acid (C22:6, ω-3; DHA). DHA has played a unique role during evolution of the modern hominid brain [20] and adequate intake of ω-3 polyunsaturated fatty acids (PUFA), especially of DHA, has probably been very important for the developing brain of early hominids at the African savannah. But it also plays a dominant role, especially in the perinatal period, when it is supplied by the mother to the foetus through the placenta, and then to the new-born through lactation. Maintenance of adequate DHA intake into old age is crucial to prevent the onset of brain dysfunctions [59].
A continuous supply of FA to the Central Nervous System (CNS) and their replacement in CNS membranes take place throughout life, a major role being played by the liver in the processes involving FA metabolism and delivery to peripheral organs, including the brain [98]. The CNS is also unique because it does not use Linoleic acid (C18:2, ω-6; LA) and α-Linolenic acid (C18:3, ω-3; ALA) taken up from the food but only their desaturated and elongated Long-Chain PUFA derivatives [82].
PUFAs in the brain comprise over 90% of Docosahexaenoic acid ≈DHA (C22:6, ω-3; DHA) and of Arachidonic acid (C20:4, ω-6; ARA) and its elongation product Docosatetraenoic acid (C22:4, ω-6; DTA [151].
In humans, Docosahexaenoic acid (C22:6, ω-3, DHA) is either obtained from the diet or may be synthesized in small amounts from Eicosapentaenoic acid (EPA, 20:5, ω-3) via Docosapentaenoic acid (DPA, 22:5 ω-3).
For years, it was generally accepted that the biosynthesis of PUFAs takes place in the endoplasmic reticulum, which is also the most important site for phospholipid biosynthesis. However, ALA is converted, via elongation and desaturation reactions, in microsomes to Tetrasahexaenoic acid (C24:6, ω-3; THA) which is then β-oxidized in peroxisomes to form the final product DHA, a pathway which is called Sprecher’s shunt [61-66].
The only study comparable with our C57bl6 High-fat diet obese mouse model on 22% bovine lard [12] is that of [47] with domestic cats given a diet consisting of meat with deuterium-labelled FA tracers for a period of six months. It major outcomes were: a). Firstly, the confirmation of the Sprecher’s shunt as the biochemical pathway towards the for brain-development and -functioning so ‘mysterious’ Docosahexaenoic acid (C22:6, ω-3; DHA); b). Secondly, VLCFA accumulation in the brain for other PUFAs like Eicosapentaenoic acid (C20:5, ω-3; EPA) and its intermediate Docosapentaenoic acid (C22:5, ω-3; DPA) [47], which probably functions as a buffer between EPA and DHA [75].
Our ‘ABS hypothesis’, described in this review in response to the challenge by the proponents of the APH, provides the required biochemical model based solely on molecular building materials from the African savannah supporting the SDL hypothesis and explaining the encephalization of the human brain (Figure 27).
Acknowledgments
Floris Schouten is kindly acknowledged for drawing several Figures. Dr. Hans Lammers is kindly acknowledged for critical reading the manuscript and helpful suggestions. Furthermore, I’m very grateful to Jaap & Marita van Meerveld for continuous help and support.
References
- Gonder MK, Mortensen HM, Reed FA, de Sousa A, Tishkoff SA. Whole- mtDNA genome sequence analysis of ancient African lineages. Mol Biol Evol. 2007; 24: 757–768.
- Stringer C. Human evolution: Out of Ethiopia. Nature. 2003; 423: 692-695.
- Mellars P. A new radiocarbon revolution and the dispersal of modern humans in Eurasia. Nature. 2006; 439: 931-935.
- Meredith M. Born in Africa: The Quest for the Origins of Human Life New York: Public Affairs. 2011; ISBN 1-58648-663-2.
- Wood B, Collard M. The human genus. Science. 1999; 284: 65-71.
- Herculano-Houzel S. ‘Hominin Evolution: Estimates of Numbers of Brain Neurons in Prehistoric Homo’; Chapter 1: 2-12; Book: “Brain Evolution”; The Human Nervous System, third edition; Eds. JK Mai & G Paxinox, 2012; Elsevier Inc.
- Rightmire GP. Homo erectus and Middle Pleistocene hominins: brain size, skull form, and species recognition. J Hum Evol. 2013; 65: 223-253.
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiat. 2007; 62: 847– 855.
- van Ginneken V. Where Darwin neglected to explain the human brain encephalization: 1). Ecological arguments supporting the Savannah Dryland (SDL) Hypothesis. ES Journal of Neurology. 2020; 1: 1005.
- Aremu OT, Onadeko BA, Ola-Adams BA, Inah EI. Population parameters and Biomass of African Buffalo (Syncerus caffer) in Kainji Lake, National Park, Nigeria. Journal of Applied Sciences. 2007; 7: 1809-1812.
- van Ginneken V, Verheij E, Poelmann R, Ramakers R, van Dijk KW, Ham L Voshol P, et al. Metabolomics (liver and blood profiling) in a mouse model in response to fasting: a study at hepatic steatosis. Biochimica Biophysica Acta. 2007; 1771: 1263-1270.
- van Ginneken V, de Vries E, Verheij E, van der Greef J. ‘Brain steatosis’ in an obese mouse model during cycles of Famine and Feast: the underestimated role of fat (WAT) in brain volume formation. Integr Mol Med. 2017; 4: 1-6.
- Williams MF. Primate encephalization and intelligence. Med. Hypotheses. 2002; 58: 284–290.
- van Ginneken V, van Meerveld A, Wijgerde T, Verheij E, de Vries E, van der Greef J. Hunter-prey correlation between migration routes of African buffaloes and early hominids: Evidence for the “Out of Africa” hypothesis. J Mol Med. 2017; 4: 1-5.
- van Ginneken V, van Meerveld A, Verheij E, van der Greef J. On the Futile Existence of DHA, None of EPA and the Predominant Role of the Triacylglycerols (TGs) in the Post Mortem Human Brain: An LCMS Study with Evolutionary Implications. J Bioanal Biomed. 2017; 9: 152-158.
- Aiello LC. Brains and guts in human evolution: The Expensive Tissue Hypothesis. Current Anthropol. 1997; 36: 199-221.
- Parker ST, Gibson KR. A developmental model for the evolution of language and intelligence in early hominids. Behav Brain Sci. 1979; 2: 367-407.
- Gibson KR. Cognition brain size and the extraction of embedded food resources. In: Primate Ontogeny Cognition and Social Behaviour (Else JG & Lee PC eds) Cambridge University Press Cambridge. 1986; 93-105.
- Leonard WR, Robertson ML. Nutritional requirements and human evolution: A bioenergetics model. Am J Hum Biol. 1992; 4: 179-195.
- Crawford MA, Gale MM, Woodford MH. Linoleic acid and Linolenic acid Elongation Products in Muscle Tissue of Syncerus caffer and Other Ruminant Species. Biochem J. 1969; 115: 25-27.
- Broadhurst CL, Wang Y, Crawford MA, Cunnane SC, Parkington JE, Schmidt WF. Brain-specific lipids from marine, lacustrine, or terrestrial food resources: potential impact on early African Homo sapiens. Comparative Biochemistry and Physiology Molecular Biology Part B. 2002; 131: 653-673.
- van Ginneken V, Helsper J, de Visser W, van Keulen H, Brandenburg W. Polyunsaturated fatty acids in various macro algae species from North Atlantic and Tropical Seas. Lipids in Health and Disease Jun. 2011; 22; 104.
- Crawford MA, Bloom M, Cunnane S, Holmsen H, Ghebremeskel K, Parkington J, et al. Docosahexaenoic acid and cerebral evolution. World Rev Nutr Diet. 2001; 88: 6–17.
- Crawford MA. Cerebral Evolution. Nutrition and Health. 2002; 16: 29–34.
- Carlson BA. Kingston JD. Docosahexaenoic acid biosynthesis and dietary contingency: Encephalization without aquatic constraint. Am J Hum Biol. 2007; 19: 585- 588.
- Darwin C. ”On the Origin of Species”; [edited by Quammen D (2011), Sterling Publishing, New York]; ISBN . 1859; 978-1-4027-8959-5; 544.
- Darwin C. “The Descent of Man, and Selection in Relation to Sex”; Publisher: John Murray; Publication date: 24 February 1871; Media Type: Print (hardback); two 450-page volumes. 1871.
- Domínguez-Rodrigo M. Is the “Savanna Hypothesis” a Dead Concept for Explaining the Emergence of the Earliest Hominins? Current Anthropology. 2014; 55: 59-81.
- Tuomisto H, Tuomisto M, Tuomisto JT. How scientists perceive the evolutionary origin of human traits: Results of a survey study. Ecology and Evolution. 2018; 8: 3518–3533.
- Hardy A. Was Man more Aquatic in the Past? New Scientist. 1960; 7: 642-645.
- Brenna JT, Carlson SE. Docosahexaenoic acid and human brain development: evidence that a dietary supply is needed for optimal development. J Hum Evol. 2014; 77: 99-106.
- Foley R, Lahr MM. The role of “the aquatic” in human evolution: Constraining the aquatic ape hypothesis. Evolutionary Anthropology: Issues, News, and Reviews. 2014; 23: 56–59.
- van Ginneken VJT. A stochiometric model for Human Brain Evolution. EJPMR. 2019; 6: 221-230.
- Marean CW, Bar-Matthews M, Bernatchez J, et al. Early human use of marine resources and pigment in South Africa during the Middle Pleistocene. Nature. 2007; 449: 905–908.
- Cortes-Sanchez M, Morales-Muňiz A, Simon-Vallejo MD, et al. Earliest known use of marine resources by Neanderthals. PLoS ONE 6: e24026. 2011.
- Braun DR, Harris, JWK, Levin NE, McCoy JT, Herries AIR, et al. Early hominin diet included diverse terrestrial and aquatic animals 1.95 Ma in East Turkana, Kenya. Proceedings of the National Academy of Sciences. 2010; 107; 10002–10007.
- Brooks AS, Helgren DM, Cramer JS, et al. Dating and context of three Middle Stone Age sites with bone points in the Upper Semliki Valley, Zaire. Science. 1995; 268: 548–553.
- O’Connor S, Ono R, Clarkson C. Pelagic fishing at 42,000 years before the present and the maritime skills of modern humans. Science. 2011; 334: 1117–1121.
- Brumm A, Jensen M, van den Bergh GD, et al. Hominins on Flores, Indonesia, by one million years ago. Nature. 2010; 464: 748–752.
- WoldeGabriel G, Ambrose SH, Barboni D, et al. The geological, isotopic, botanical, invertebrate, and lower vertebrate surroundings of Ardipithecus ramidus. Science. 2009; 326: 65.
- van Ginneken V. Multiple “Genetic Bottleneck Theory” of Humans and the House Mouse via Chilling Enzyme ∆12-Desaturase. Gastroenterology & Hepatology International Journal. 2019; 4.
- Templeton A. Out of Africa again and again. Nature. 2002; 416: 45–51.
- Kitano H. Systems Biology: a brief overview. Science. 2002; 295: 1662-1664.
- Dennis EA. Lipidomics joins the omics evolution. Proceedings of the National Academy of Sciences of the United States of America. 2009; 106; 2089–2090.
- Ridgway N, McLeod RS (editors). Book 6th Edition: Biochemistry of Lipids, Lipoproteins and Membranes; ISBN: 978-0-444-63438-2; Elsevier Science; 2016; 612.
- Kuipers R, Luxwolda M, Offringa P, et al. Foetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot Essent Fatty acids. 2012; 86: 13-20.
- Pawlowski R, Barnes A, Salem N Jr. Essential fatty acid metabolism in the feline: relationship between liver and brain production of long-chain polyunsaturated fatty acids. J Lipid Res. 1994; 35: 2032-2040.
- Megaze A, Balakrishnan M, Belay G. Current population estimate and distribution of the African buffalo in Chebera Churchura National Park, Ethiopia. African Journal of Ecology. 2017; 56: 12–19.
- Aremu OT, Onaadeko BA, Ola-Adams BA, Inah EI. Population parameters and Biomass of African Buffalo (Syncerus caffer) in Kainji Lake, National Park, Nigeria. Journal of Applied Sciences. 2007; 7: 1809-1812.
- Stringer CB. Evolution of Early Humans. In: Steve Jones, Robert Martin, David Pilbeam. The Cambridge Encyclopedia of Human Evolution. Cambridge: Cambridge University Press; ISBN 978-0-521-32370-3; 1994; 242.
- Haile-Selassie Y, Melilla SM, Vazzana A, Benazzi S, Ryan TM. A 3.8-million-year-old hominin cranium from Woranso-Mille, Ethiopia. Nature. 2019.
- Kimbel WH, Lockwood CA. Endocranial Capacity of Early Hominids. Science. 1999; 283: 9.
- Pearce E, Stringer C, Dunbar RIM. New insights into differences in brain organization between Neanderthals and anatomically modern humans. Proc R Soc B. 2013; 280.
- Parent A, Carpenter MB. "Ch. 1". Carpenter's Human Neuroanatomy. Williams & Wilkins. 1995; ISBN 978-0-683-06752-1.
- Bradbury J. Docosahexaenoic acid (DHA): an ancient nutrient for the modern human brain. Nutrients. 2011; 3: 529–554.
- Stringer CB. The dates of Eden. Nature. 1998; 331: 565-566.
- Rolfes SR, Pinna K, Whitney E. Understanding Normal and Clinical Nutrition, Eight Edition, Wadsworth, Cengage Learning. 2009; 6: 925.
- Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nature reviews. Immunology. 2015; 15: 511–523.
- Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. The Journal of Pediatrics. 2003; 143: 1–8.
- Kaur N, Chugh V, Gupta AK. Essential fatty acids as functional components of foods- a review. Journal of Food Science and Technology. 2012; 51: 2289–2303.
- Ferdinandusse S, Denis S, Mooijer PAW, Zhang Z, Reddy JK, Spector AA, et al. Identification of the peroxisomal β-oxidation enzymes involved in the biosynthesis of docosahexaenoic acid. J Lipid Res. 2001; 42: 1987-1995.
- Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Frontiers in aging neuroscience. 2015; 7.
- De Caterina R, Basta G. Ω-3 Fatty acids and the inflammatory response – biological background. European Heart Journal Supplements 3 (Supplement D): 2001; D42–D49.
- Voss A, Reinhart M, Sankarappa S, Sprecher H. The metabolism of 7,10,13,16,19-docosapentaenoic acid to 4,7,10,13,16,19-docosahexaenoic acid in rat liver is independent of a ∆4-desaturase. The Journal of Biological Chemistry. 1991; 266: 19995–20000.
- Bederska-Lojewska D, Orczasvska-Dudek S, Pieszka M. Metabolism of Arachidonic acids, its concentration in animal products and influence on inflammatory processes in the human body: a review. Annals of Animal Science. 2013; 13.
- Metherel AH, Lacombe R, Chouinard-Watkins R & Bazinet RP. Docosahexaenoic acid is both a product of and a precursor to tetracosahexaenoic acid in the rat. Journal of lipid research. 2019; 60: 2102.
- Crawford MA, Cunnane SC, Harbige LS. A New Theory of Evolution: Quantum Theory, in 3rd International Congress on Essential Fatty acids" and Eicosanoids, (Sinclair, A.J., and Gibson, R. eds) AOCS Press, Adelaide: 1993; 87-95.
- van Wieren SE. Digestive strategies in ruminants and nonruminants; PhD- thesis, Agricultural University Wageningen, Netherlands; 1996; 611-614; 191.
- Macandza VA, Owen-Smith N, Cross PC. Forage selection by the African buffalo in the late dry season in two landscapes. South African Journal of Wildlife Research. 2004; 34: 113-121.
- Williams PM, Bowden BN. Triglyceride metabolism in germinating Andropogon gayannus seeds. Phytochemistry. 1973; 12: 2821-2827.
- Knight TW, Knowles S, Death AF, West J, Agnew M, Morris CA, et al. Factors affecting the variation in fatty acid concentrations in lean beef from grass-fed cattle in New Zealand and the implications for human health. N Z J Agr Res. 2003; 46: 83–95.
- Crawford MA, Gale MM, Woodford MH, Casped NM. Comparative studies on fatty acid composition of wild and domestic meats. International Journal of Biochemistry. 970; 1: 295–305.
- Jenkins TC, Wallace RJ, Moate PJ, Mosley EE. Board-invited review: Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. Journal of Animal Science. 2008; 86: 397-412.
- Söderberg M, Edlund MC, Kristensson K, Dallner G. Fatty acids composition of brain phospholipids in aging and Alzheimer‘s disease. Lipids. 1991; 26: 421-425.
- Drouin G, Rioux V, Legrand P. The n-3 docosapentaenoic acid (DPA): A new player in the n-3 long chain polyunsaturated fatty acid family. Biochemie. 2019; 159: 36-48.
- Shrestha P, Zhou XR, Vibhakaran Pillai S, Petrie J, de Feyter R, Singh S. Comparison of the Substrate Preferences of ω3 Fatty acid Desaturases for Long Chain Polyunsaturated Fatty acids. International journal of molecular sciences. 2019; 20: 3058.
- Prigge MJ, Bezanilla M. Evolutionary crossroads in developmental biology: Physcomitrella patens. Development. 2010; 137: 3535–3543.
- Park WJ, Kothapalli KS, Lawrence P, Tyburczy C, Brenna JT. An alternate pathway to long-chain polyunsaturates: The FADS2 gene product Δ8- desaturates 20:2n-6 and 20:3n-3. J Lipid Res. 2009; 50: 1195–1202.
- Glaser C, Lattka E, Rzehak P, Steer C, Koletzko B. Genetic variation in polyunsaturated fatty acid metabolism and its potential relevance for human development and health. Maternal & Child Nutrition. 2011; 7: 27–40.
- Ma X, Yu J, Zhu B, Pan K, Pan J, Yang G. Cloning and characterization of a delta-6 desaturase encoding gene from Nannochloropsis oculata. Chinese Journal of Oceanology and Limnology. 2011; 29: 290–296.
- Carreño D, Toral PG, Pinloche E, Belenguer A, Yáñez-Ruiz DR, Hervás G, et al. Rumen bacterial community responses to DPA, EPA and DHA in cattle and sheep: A comparative in vitro study. Scientific reports. 2019; 9: 11857.
- Abedi E, Sahari MA. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Science & Nutrition. 2014; 2: 443–463.
- Sakuradani E, Kamada N, Hirano Y, Nishihara M, Kawashima H, Akimoto K. Production of 5,8,11-eicosatrienoic acid by a Δ5 and Δ6 desaturation activity- enhanced mutant derived from a Δ12 desaturation activity-defective mutant of Mortierella alpina 1S–4. Appl Microbiol Biotechnol. 2002; 60: 281–287.
- Certik M, Sakuradani E, Shimizu S. Desaturase defective fungal mutants: useful tools for the regulation and overproduction of polyunsaturated fatty acids. Trends Biotechnol. 1998; 16: 500–505.
- Certik M, Shimizu S. Biosynthesis and Regulation of Microbial Polyunsaturated Fatty Acid Production. Journal of Bioscience and Bioengineering. 1999; 87: 1-14.
- Martins D, Custódio L, Barreira L, Pereira H, Ben-Hamadou R, Varela J, et al. Alternative Sources of n-3 Long-Chain Polyunsaturated Fatty acids in Marine Microalgae. Marine Drugs. 2013; 11: 2259–2281.
- Kramer JKG, Parodi PW, Jensen RG, Mossoba MM, Yurawecz MP, Adlof RO. Rumenic acid: A proposed common name for the major conjugated linoleic acid isomer found in natural products. Lipids. 1998; 33: 835–835.
- Bauman DE, Baumgard LH, Corl BA, Griinari JM. Biosynthesis of conjugated linoleic acid in ruminant. Proceedings of the American Society of Animal Science. 1999.
- Padre R das G, Aricetti JA, Moreira FB, Mizubuti IY, do Prado IN, Visentainer JV, et al. Fatty acid profile, and chemical composition of Longissimus muscle of bovine steers and bulls finished in pasture system. Meat Science. 2006; 74: 242–248.
- Vahmani P, Mapiye C, Prieto N, Rolland DC, McAllister TA, Aalhus JL, Dugan MER. The scope for manipulating the polyunsaturated fatty acid content of beef: a review. Journal of Animal Science and Biotechnology. 2015; 29.
- Turpeinen AM, Mutanen M, Aro A, Salminen I, Basu S, Palmquist DL, Griinari JM. Bioconversion of vaccenic acid to conjugated linoleic acid in humans. The American Journal of Clinical Nutrition. 2002; 76: 504–510.
- Belury MA. Inhibition of Carcinogenesis by Conjugated Linoleic acid: Potential Mechanisms of Action. The Journal of Nutrition. 2002; 132: 2995–2998.
- Cho HP, Takamura MT, Clarke SD. Cloning, expression, and nutritional regulation of the mammalian Δ6 desaturase. J Biol Chem. 1999; 274: 471–477.
- Marquardt A, Stohr H, White K, Weber BH. cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics. 2000; 66: 175–183.
- Chilliard Y, Glasser F, Ferlay A, Bernard L, Rouel J, Doreau M. Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. Europ J Lipid Sci Technol. 2007; 109: 828-854.
- Scollan N, Hocquette JF, Nuernberg K, Dannenberger D, Richardson I, Moloney A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006; 74: 17- 33.
- Nakamura MT, Nara TY. Structure, function, and dietary regulation of Δ6, Δ5, and Δ9 desaturases. Annu. Rev. Nutr. 2004; 24: 345–376.
- Salway JG. Metabolism at a Glance. Blackwell Publishing LTD, ISBN-978-1- 4051-0716-7. 2014; 125.
- Roman C, Carriere F, Villeneuve P, Pina M, Millet V, Simeoni U, Sarles J. Quantitative and Qualitative Study of Gastric Lipolysis in Premature Infants: Do MCT-Enriched Infant Formulas Improve Fat Digestion? Pediatric Research. 2007; 61: 83–88.
- Werner A, Kuipers F, Verkade HJ. Fat Absorption and Lipid Metabolism in Cholestasis. In: Madame Curie Bioscience Database. Austin (TX): Landes Bioscience; 2000-2013.
- Ackman RG, Burgher RD. Cod Liver Oil: Component Fatty acids as Determined by Gas-Liquid Chromatography; Journal of the Fisheries Research Board of Canada. 1964; 21: 319-326.
- Griinari JM, Corl BA, Laly SH, Chourinard PY, Bauman BE. Conjugated Linoleic Acid is synthesized endogenously in lactating dairy cows by delta 9 desaturase. J Nutr. 2000; 130: 2285-2291.
- Bauman DE, Baumgard LH, Corl BA, Griinari JM. Biosynthesis of conjugated linoleic acid in ruminants. Journal of Animal Science. 2000; 77.
- van Ginneken V, Verheij E, van der Greef J. Impact of the ‘wrong’ fats on the composition of the heart muscle in an obese High Fat diet (HF-diet) induced Insulin Resistant C57bl6 mouse model following a Lipidomics Systems Biology LCMS approach. EJPMR. 2019; 6: 243-262.
- Greupner T, Kutzner L, Pagenkopf S, Kohrs H, Hahn A, Schebb NH, Schuchardt JP. Effects of a low and a high dietary LA/ALA ratio on long-chain PUFA concentrations in red blood cells. Food & Function. 2018.
- Szefel J, Kruszewski WJ, Sobczak E. Factors influencing the eicosanoids synthesis in vivo. BioMed research international. 2015; 690692.
- Delplanque B, Gibson R, Koletzko B, Lapillonne A, Strandvik B. Lipid quality in infant nutrition: current knowledge and future opportunities, J Pediatr Gastroenterol Nutr. 2015; 61.
- Li J, Yin H, Bibus DM, Byelashov OA. The role of Omega-3 docosapentaenoic Acid in pregnancy and early development, Eur J Lipid Sci. Technol. 2016.
- Schipper L, Dijk Gv, Beek EMvd. Milk lipid composition and structure; The relevance for infant brain development. OCL. 2020; 27: 5.
- Rahmawaty S, Charlton K, Lyons-Wall P, Meyer BJ. Dietary intake and food sources of EPA, DPA and DHA in Australian children, Lipids. 2013; 48.
- Gregory MK, Gibson RA, Cook-Johnson RJ, Cleland LG, James MJ. Elongase reactions as control points in Long-Chain polyunsaturated fatty acid synthesis. PLoS One. 2011; 6.
- Fei-Guo X, Sinclair AJ, Kaur G, Li D. Differential effects of EPA, DPA and DHA on cardio-metabolic risk factors in high-fat diet fed mice, Prostaglandins Leukot. Essent Fatty acids. 2017.
- Kaur G, Begg DP, Barr D, Garg M, Cameron-Smith D, Sinclair AJ. Short-term docosapentaenoic acid (22:5n-3) supplementation increases tissue docosapentaenoic acid, DHA and EPA concentrations in rats. Br J Nutr. 2010; 103: 32e37.
- Gotoh N, Nagao K, Onoda S, Shirouchi B, Furuya R, et al. Effects of three different highly purified n-3 series highly unsaturated fatty acids on lipid metabolism inC57BL/KsJ-dbl db mice. J Agric Food Chem. 2009; 57: 11047e11054.
- Alvarez RA, Aguirre GD, Acland GM, Anderson RE. Docosapentaenoic acid is converted to docosahexaenoic acid in the retinas of normal and prcd affected miniature poodle dogs, Investig. Ophthalmol Vis Sci. 1994; 35: 402e408.
- Park HG, Park WJ, Kothapalli KS, Brenna JT. The fatty acid desaturase 2 (FADS2) gene product catalyses Δ4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J. 2015; 29: 3911–3919.
- Miller E, Kaur G, Larsen A, Loh SP, Linderborg K, Weisinger HS, et al. A short-term n-3 DPA supplementation study in humans. Eur J Nutr. 2013; 52: 895e904.
- Karupaiah T, Sundram K. Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: a review of their nutritional implications. Nutrition & metabolism. 2007; 4: 16.
- Wang TY, Liu M, Portincasa P, Wang DQ. New insights into the molecular mechanism of intestinal fatty acid absorption. European journal of clinical investigation. 2013; 43: 1203–1223.
- Ye Z, Cao C, Li R, Cao P, Li Q, Liu Y. Lipid composition modulates the intestine digestion rate and serum lipid status of different edible oils: a combination of in vitro and in vivo studies. Food & Function. 2019.
- Doyle E. Saturated Fat and Beef Fat as Related to Human Health: A Review of the Scientific Literature FRI Briefings Food Research Institute University of Winconsin Madison WI. 2014; 53706; 28.
- Eichmann TO, Lass A. DAG tales: the multiple faces of diacylglycerol--stereochemistry, metabolism, and signalling. Cellular and molecular life sciences: CMLS. 2015; 72: 3931–3952.
- Bruckner G. Fatty acids and cardiovascular disease. In: Chow CK (ed.), Fatty acids in Foods and Their Health Implications. Marcel Dekker, Inc. New York. 2000; 843-846.
- Bracco U. Effect of triglyceride structure on fat absorption. Am J Clin Nutr. 1994; 60: S1002–S1009.
- Livesey G. The absorption of Stearic acid (C18:0; ω-9; SA) from triacylglycerols: an inquiry and analysis. Nutr Res Rev. 2000; 13: 185–214.
- Summers LKM, Fielding BA, Herd SL, Ilic V, Clark ML, Quinlan PT, Frayn KN. Use of structured triacylglycerols containing predominantly stearic and oleic acids to probe early events in metabolic processing of dietary fat. J Lipid Res. 1999; 40: 1890–1898.
- Yli-Jokipii K, Kallio H, Schwab U, Mykkänen H, Kurvinen JP, Savolainen MJ, Tahvonen R. Effects of palm oil and trans-esterified palm oil on chylomicron and VLDL triacylglycerol structures and postprandial lipid response. J Lipid Res. 2001; 42: 1618–1625.
- Abia R, Pacheco YM, Perona JS, Montero E, Muriana FJG, Ruiz- Gutiérrez V. The metabolic availability of dietary triacylglycerols from two high oleic oils during the postprandial period does not depend on the amount of oleic acid ingested by healthy men. J Nutr. 2001; 131: 59–65.
- Berry SEE, Sanders TAB. Influence of triacylglycerol structure of Stearic acid (C18:0; ω-9; SA) -rich fats on postprandial lipaemia. Proc Nutr Soc. 2005; 64: 205–212.
- Mattson FH, Lutton ES. The specific distribution of fatty acids in the glycerides of animal and vegeTable fats. J Biol Chem. 1958; 233: 868–871.
- Brockerhoff H, Yurkowski M. Stereospecific analyses of several vegeTable fats. J Lipid Res. 1966; 7: 62–64.
- Brockerhoff H, Hoyle RJ, Wolmark N. Positional distribution of fatty acids in triglycerides of animal depot fats. Biochim Biophys Acta. 1966; 116: 67–72.
- Small DM. The effects of glyceride structure on fat absorption and metabolism. Ann Rev Nutr. 1991; 11: 412–434.
- Padley FB, Gunstone FD, Harwood JL. Occurrence and characteristics of oils and fats. In: Gunstone FD, Harwood JL, Padley FB, editor. The Lipid Handbook. 2. London: Chapman & Hall. 1994; 47–223.
- Nawar WW. Lipids. In: Fennema OR, editor. Food Chemistry. 3. New York: Marcel Dekker Inc. 1996; 225–319.
- Berry SEE. Triacylglycerol structure and interesterification of palmitic and Stearic acid (C18:0; ω-9; SA) -rich fats: an overview and implications for cardiovascular disease. Nutrition Research Reviews. 2009; 22.
- Knopp RH, Retzlaff B, Walden C, Fish B, Buck B, McCann B. One-year effects of increasingly fat-restricted, carbohydrate-enriched diets on lipoprotein levels in free-living subjects. Proc Soc Exp Biol Med. 2000; 225: 191–199.
- Glatz JFC, Luiken JJFP, Bonen A. Membrane Fatty acid Transporters as Regulators of Lipid Metabolism: Implications for Metabolic Disease. Physiol Rev. 2010; 90: 367–417.
- Marin-Valencia I, Good LB, Ma Q, Malloy CR, Pascual JM. Heptanoate as a neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain. J Cereb Blood Flow Metab. 2013; 33: 175-182.
- O’Brien JS, Sampson EL. Lipid composition of the normal human brain: gray matter, white matter, and myelin. J Lipid Res. 1965; 6: 537–544.
- Spector AA. Plasma free fatty acid and lipoproteins as sources of poly- unsaturated fatty acid for the brain. J Mol Neurosci. 2001; 16: 159–165.
- Benatti P, Peluso G, Nicolai R, Calvani M. Polyunsaturated Fatty Acids: Biochemical, Nutritional and Epigenetic Properties. Journal of the American College of Nutrition. 2004; 23: 281–302.
- Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. Int J Dev Neurosci. 2000; 18: 383–399.
- Martinez M, Mougan I. Fatty acid composition of human brain phospholipids during normal development. J Neurochem. 1998; 71: 2528–2533.
- Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015; 7: 52.
- Duncan RE, Bazinet RP. Brain arachidonic acid uptake and turnover: implications for signalling and bipolar disorder. Curr Opin Clin Nutr Metab Care. 2010; 13: 130–138.
- Hadley KB, Ryan AS, Forsyth S, Gautier S, Salem N Jr. The Essentiality of Arachidonic Acid in Infant Development. Nutrients. 2016; 8: 216.
- Robson LG, Dyall S, Sidloff D, Michael-Titus AT. Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurones throughout development and in aged animals. Neurobiology of Aging. 2010; 31: 678– 687.
- Foot M, Cruz TF, Clandinin MT. Influence of dietary fat on the lipid composition of rat brain synaptosomal and microsomal membranes. The Biochemical journal. 1982; 208: 631–640.
- Carrié I, Clément M, de Javel D, Francès H, Bourre JM. Specific phospholipid fatty acid composition of brain regions in mice: effects of n–3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J Lipid Res. 2000; 41: 465–472.
- Novak EM, Dyer RA, Innis SM. High dietary omega-6 fatty acids contribute to reduced docosahexaenoic acid in the developing brain and inhibit secondary neurite growth. Brain Res. 2008; 1237: 136–145.
- Katakura M, Hashimoto M, Okui T, Shahdat HM, Matsuzaki K, Shido O. Omega-3 Polyunsaturated Fatty Acids Enhance Neuronal Differentiation in Cultured Rat Neural Stem Cells. Stem Cells International. 2013: 1–9.
- Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr. 1992; 120: S129–S138.
- Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Research Bulletin. 2001; 56: 79–85.
- Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Frontiers in neuroendocrinology. 2014; 35: 473–493.
- Herbert AL, Monk KR. Advances in myelinating glial cell development. Current opinion in neurobiology. 2017; 42: 53–60.
- Carlson SE, Colombo J. Docosahexaenoic Acid and Arachidonic Acid Nutrition in Early Development. Advances in pediatrics. 2016; 63: 453–471.
- Kihara A. Very long-chain fatty acids: elongation, physiology and related disorders. Journal of Biochemistry. 2012; 152: 387–395.
- Sassa T, Kihara A. Metabolism of very long-chain Fatty acids: genes and pathophysiology. Biomolecules & therapeutics. 2014; 22: 83–92.
- Kemp S, Theodoulou FL, Wanders RJA. Mammalian peroxisomal ABC transporters: from endogenous substrates to pathology and clinical significance. Br J Pharmacol. 2011; 164: 1753-1766.
- Hishikawa D, Valentine WJ, Iizuka-Hishikawa Y, Shindou H, Shimizu T. Metabolism and functions of docosahexaenoic acid-containing membrane glycerophospholipids. FEBS letters. 2017; 591: 2730–2744.
- Hussein N, Ah-Sing E, Wilkinson P, Leach C, Griffin BA, Millward DJ. Long-chain conversion of [13C] linoleic acid and a-linolenic acid in response to marked changes in their dietary intake in men. J Lip Res. 2005; 46: 269-280.
- Moore SA, Hurt E, Yoder E, Sprecher H, Spector AA. Docosahexaenoic acid synthesis in human skin fibroblasts involves peroxisomal retro- conversion of tetracosahexaenoic acid. J Lipid Res. 1995; 36: 2433-2443.
- Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prost Leukot Essent Fatty acids. 2007; 77: 251-261.
- Serhan CN. Novel eicosanoid and docasanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005; 8: 115-121.
- Caruso D, Rise´ P, Galella G, Regazzoni C, Toia A, Galli G, et al. Formation of 22 and 24 carbon 6-desaturated fatty acids from exogenous deuterated arachidonic acid is activated in THP-1 cells at high substrate concentrations. FEBS Lett. 1994; 343: 195-199.
- Galli C, Risé P, Marangoni F. Fate of exogenous arachidonic acid in THP-1 cells: Incorporation in cell lipids and conversion to other ω-6 fatty acids. Prostaglandins, Leukotrienes and Essential Fatty acids. 1995; 52: 103–106.
- Banni S. Conjugated linoleic acid metabolism. Curr. Opin. Lipidol. 2002; 13: 261-266.
- Banni S, Petroni A, Blasevich M, Carta G, Cordeddu L, Murru E, et al. Conjugated linoleic acids (CLA) as Precursors of a distinct family of PUFA. Lipids. 2004; 39: 1143-1146.
- Inoue T, Hashimoto M, Katakura M, Hossain S, Matsuzaki K, Shido O. Effect of chronic administration of arachidonic acid on the performance of learning and memory in aged rats. 2020; 630.
- Hanna VS, Hafez E. Synopsis of arachidonic acid metabolism: A review. Journal of advanced research. 2018; 11: 23–32.
- Ciappolino V, Mazzocchi A, Botturi A, Turolo S, Delvecchio G, Agostoni C, et al. The Role of Docosahexaenoic acid (DHA) on Cognitive Functions in Psychiatric Disorders. Nutrients. 2019; 11: 769.
- Chen CT, Liu Z, Ouellet M, Calon F, Bazinet RP. Rapid β- oxidation of eicosapentaenoic acid in mouse brain: an in-situ study. Prostaglandins Leukot Essent Fatty acids. 2009; 80: 157-163.
- Chen CT, Liu Z, Bazinet RP. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: an intracerebroventricular study. J Neurochem. 2011; 116: 363-373.
- Umhau JC, Zhou W, Carson RE, Rapoport SI, Polozova A, Demar J, et al. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J Lipid Res. 2009; 50: 1259-1268.
- Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr. 2009; 28: 525-542.
- Sato M, Adan Y, Shibata K, Shoji Y, Sato H, Imaizumi K. Cloning of rat delta 6-desaturase and its regulation by dietary eicosapentaenoic or docosahexaenoic acid. World Rev Nutr Diet. 2001; 88: 196-199.
- Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003; 126: 1-27.
- Yurlova L, Kahya N, Aggarwal S, Kaiser HJ, Chiantia S, Bakhti M, Simons M. Self-segregation of myelin membrane lipids in model membranes. Biophysical journal. 2011; 101: 2713–2720.
- Carrié I, Clément M, de Javel D, Francès H, Bourre JM. Specific phospholipid fatty acid composition of brain regions in mice: effects of n–3 polyunsaturated fatty acid deficiency and phospholipid supplementation J Lipid Res. 2000; 41: 465-472.
- Ikemoto A, Ohishi M, Sato Y, Hata N, Misawa Y, Fujii Y, Okuyama H. Reversibility of n-3 fatty acid deficiency-induced alterations of learning behaviour in the rat: level of n-6 fatty acids as another critical factor. J Lipid Res. 2001; 42: 1655-1663.
- Anderson GJ, Neuringer M, Lin DS, Connor WE. Can Prenatal N- 3 Fatty Acid Deficiency Be Completely Reversed after Birth? Effects on Retinal and Brain Biochemistry and Visual Function in Rhesus Monkeys. Pediatric Research. 2005; 58: 865–872.
- Bakker E, Hornstra G, Blanco C. Relationship between long- chain polyunsaturated fatty acids at birth and motor function at 7 years of age. Eur J Clin Nutr. 2009; 63: 499–504.
- Bakker EC, Ghys AJA, Kester ADM, Vles JSH, Dubas JS, Blanco CE, Hornstra G. Long-chain polyunsaturated fatty acids at birth and cognitive function at 7 y of age. European Journal of Clinical Nutrition. 2003; 57: 89–95.
- Boucher O, Burden MJ, Muckle G, Saint-Amour D, Ayotte P, Dewailly E, et al. Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. The American journal of clinical nutrition. 2011; 93: 1025–1037.
- Escolano-Margarit MV, Ramos R, Beyer J, Csábi G, Parrilla-Roure M, Cruz F, Campoy C. Prenatal DHA Status and Neurological Outcome in Children at Age 5.5 Years Are Positively Associated. The Journal of Nutrition. 2011; 141: 1216–1223.
- McNamara RK, Hahn CG, Jandacek R, Rider T, Tso P, Stanford KE, Richtand NM. Selective Deficits in the Omega-3 Fatty Acid Docosahexaenoic Acid in the Post mortem Orbitofrontal Cortex of Patients with Major Depressive Disorder. Biological Psychiatry. 2007; 62: 17–24.
- Conklin SM, Runyan CA, Leonard S, Reddy RD, Muldoon MF, Yao JK. Age-related changes of n-3 and n-6 polyunsaturated fatty acids in the anterior cingulate cortex of individuals with major depressive disorder. Prostaglandins, leukotrienes, and essential fatty acids. 2010; 82: 111–119.
- Hamazaki K, Hamazaki T, Inadera H. Abnormalities in the fatty acid composition of the post-mortem entorhinal cortex of patients with schizophrenia, bipolar disorder, and major depressive disorder. Psychiatr Res. 2013; 210: 346–350.
- Price JS. Evolutionary aspects of anxiety disorders. Dialogues in clinical neuroscience. 2003; 5: 223–236.
- Arain M, Haque M, Johal L. Maturation of the adolescent brain. Neuropsychiatric disease and treatment. 2013; 9: 449-461.
- Mori TA, Burke V, Puddey IB, Watts GF, O'Neal DN, Best JD, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000; 71: 1085-1094.
- Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005; 67: 867-874.
- Narayan B, Miyashita K, Hosakawa M. Physiological Effects of Eicosapentaenoic acid (EPA) and Docosahexaenoic acid (DHA)—A Review. Food Reviews International. 2006; 22: 291–307.
- Calder PC. Omega-3 fatty acids in the inflammatory processes: from molecule to man. Biochemical Society Transactions. 2017.
- Marquardt A, Stohr H, White K, Weber BH. cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics. 2000; 66: 175–183.
- Lagarde M, Bernoud N, Brossard N, Lecerf JM. Lysophosphatidylcholine as a Preferred Carrier Form of Docosahexaenoic acid to the Brain. Journal of Molecular Neuroscience. 2001; 16: 201-204.
- Pardridge WM, Connor JD, Crawford IL. Permeability changes in the blood–brain barrier: causes and consequences, CRC Crit Rev Toxicol. 1975; 3: 159–199.
- van Ginneken V, Verheij E, Hekman M, van der Greef J. Characterization of the lipid profile post mortem for Type-2 diabetes in human brain and plasma of the elderly with LCMS-techniques: a descriptive approach of diabetic encephalopathy. Integr Mol Med. 2017; 4: 1-10.
- Benbrook CM, Davis DR, Baranski M. Enhancing the fatty acid profile of milk through forage-based rations, with nutrition modelling of diet outcomes. Food science & nutrition. 2018; 6: 681–700.
- Chilliard Y, Ferlay A, Doreau M. Contrôle de la qualité nutritionnelle des matières grasses du lait par l'alimentation des vaches laitières: Acides gras trans, polyinsaturés, Acide linoléique conjugué. Productions Animales-Paris-Institut National de la Recherche Agronomique. 2001; 14: 323-335.
- Jacome-Sosa MM, Lu J, Wang Y, Ruth MR, Proctor SD. Increased hypolipidemic benefits of cis-9, trans-11 conjugated linoleic acid in combination with trans-11 vaccenic acid in a rodent model of the metabolic syndrome, the JCR:LA-cp rat. Nutrition & metabolism. 2010; 7: 60.
- Hussain G, Schmitt F, Loeffler JP, Gonzalez de Aguilar JL. Fatting the brain: a brief of recent research. Frontiers in cellular neuroscience. 2013; 7: 144.
- Innis SM. Essential fatty acids in growth and development. Prog Lipid Res. 1991; 30: 39-101.
- van Ginneken V, Nieveen M, van den Thillart G, Addink A. Neurotransmitter levels and energy status in brain of fish species with and without the survival strategy of metabolic depression: evidence for a role of GABA in metabolic depression. Comp Biochem Physiol. 1995; 114A: 189-196.
- Service R. Will a new type of drug make memory-making easier? Science. 1994; 266: 218–219.
- Foley JF, Phadke DP, Hardy O, et al. Whole exome sequencing in the rat. BMC Genomics. 2018; 19: 487-501.
- Williard DE, Harmon SD, Kaduce TL, Preuss M, Moore SA, Robbins ME, et al. Docosahexaenoic acid synthesis from n-3 polyunsaturated fatty acids in differentiated rat brain astrocytes. J Lipid Res. 2001; 42: 1368–1376.
- Demar JC, Ma K, Chang L, Bell JM, Rapoport SI. α-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. Journal of Neurochemistry. 2005; 94: 1063–1076.
- Kitson AP, Metherel AH, Chen CT, Domenichiello AF, Trépanier MO, Berger A, Bazinet RP. Effect of dietary docosahexaenoic acid (DHA) in phospholipids or triglycerides on brain DHA uptake and accretion. The Journal of Nutritional Biochemistry. 2016; 33: 91–102.
- Bazinet RP, Laye S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014; 15: 771–785.
- Chen CT, Kitson AP, Hopperton KE, Domenichiello AF, Trépanier MO, Lin LE, et al. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Scientific Reports. 2015; 5: 15791.
- Liu JJ, Green P, John Mann J, Rapoport SI, Sublette ME. Pathways of polyunsaturated fatty acid utilization: implications for brain function in neuropsychiatric health and disease. Brain research. 2015; 1597: 220–246.
- Mitchell RW, Hatch GM. Fatty acid transport into the brain: Of fatty acid fables and lipid tails. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA). 2011; 85: 293–302.
- Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n−3 and n–6 fatty acids: estimations considering worldwide diversity. The American Journal of Clinical Nutrition. 2006; 83: 1483S–1493S.
- Igarashi M, Gao F, Kim HW, Ma K, Bell JM, Rapoport SI. Dietary n-6 PUFA deprivation for 15 weeks reduces arachidonic acid concentrations while increasing n-3 PUFA concentrations in organs of post-weaning male rats. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2009; 1791: 132–139.
- Burdge GC, Wootton SA. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr. 2002; 88: 411–420.
- Brenna JT, Salem N, Sinclair AJ, Cunnane SC. α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2009; 80: 85–91.
- Lefkowitz W, Lim SY, Lin Y, Salem N. Where Does the Developing Brain Obtain Its Docosahexaenoic Acid? Relative Contributions of Dietary α-Linolenic Acid, Docosahexaenoic Acid, and Body Stores in the Developing Rat. Pediatric Research. 2005; 57: 157–165.
- Gibson RA, Muhlhausler B, Makrides M. Conversion of linoleic acid and alpha-linolenic acid to long-chain polyunsaturated fatty acids (LCPUFAs), with a focus on pregnancy, lactation and the first 2 years of life. Matern Child Nutr. 2011; 7: 17–26.
- Bandarra NM, Lopes PA, Martins SV, Ferreira J, Alfaia CM, Rolo EA, et al. Docosahexaenoic acid at the sn-2 position of structured triacylglycerols improved n-3 polyunsaturated fatty acid assimilation in tissues of hamsters. Nutrition Research. 2016; 36: 452–463.
- Simopoulos AP. Evolutionary Aspects of Diet: The Omega-6/Omega-3 Ratio and the Brain. Molecular Neurobiology. 2011; 44: 203–215.