ES Journal of Neurology
ISSN: 2768-0606
[Review]: Where Darwin neglected to explain the human-brain encephalization: 1). Ecological arguments supporting the Savannah Dryland (SDL) hypothesis
Review article
- Vincent van Ginneken*
- Blue-Green Technologies, Heelsum, Netherlands
- *Corresponding author: Vincent van Ginneken, (PhD-1, PhD-2, MSc), Blue-Green Technologies, Heelsum, Netherlands, Email: vvanginneken@hotmail.com
- Received: Feb 20, 2020; Accepted: Feb 27, 2020; Published: Feb 29, 2020
- Copyright: 2020 © Vincent van Ginneken. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Charles Darwin neglected to explain the overgrown human brain encephalization because he had no access to modern laboratory equipment like lipidomics based LC-MS. Darwin himself was a supporter of the Savanna Dry Land (SDL) hypothesis - with the Aquatic Phase Hypothesis (APH) as its counterpart - postulating that the African savannah could supply those nutritional elements and other chemical compounds essential to the explanation of the uniqueness of the human brain with its overgrown neocortex. Here we will present all evolutionary ingredients required to explain the excessive brain growth from Homo habilis (2.4 million to 1.4 million years ago with a skull capacity of around 600 cm3), towards Homo erectus (skull capacity ≈850 cm3), probably by the invention of tools and weapons for hunting. With improving tools, more meat became available in combination with the socializing aspect of hunting (communication, language, social hierarchy). Increased meat consumption probably resulted in exponential human brain growth over the last 75,000 years towards that of modern Homo sapiens (averages brain volumes about 1250 cm3-1500 cm3). We hypothesize that the tremendous herds of the ancestors of the ruminant African buffalo (Syncerus caffer) and the biochemical composition of their products (meat & lard) formed the basis and supplied the biochemical products to explain the growth spurt of the human brain in the late Pleistocene. Another finding that supports this theory was the similarity of migration routes of early African bovines -ancestors of Syncerus caffer (based on mitochondrial DNA studies)- and early hominids (hunter-prey correlation) which provided supportive evidence for the “Out of Africa” hypothesis. So, based on these findings, we postulate the ‘African Buffalo Savannah hypothesis’ (‘ABS hypothesis’) which proposes that the availability of the meat and bovine lard of early African buffalo herds on the African savannah in the Pleistocene present the natural selection traits in evolution explaining the excessive brain growth (encephalization) of the early hominids. The use of improved tools during the hunt in combination with a dietary change towards meat can therefore be considered as a ‘prime mover’ in brain evolution. We stipulate that evolution has no purpose of itself but is based on a coincidence of circumstances, which also applies to the ‘mysteries of mysteries’, the overgrown neocortex of Homo sapiens. By a tremendously long series of ‘eatings’ and ‘breedings’ in hominid evolutionary history, those evolutionary traits were selected which ultimately resulted in the ‘overgrown human brain’ of Homo sapiens. Finally, cave paintings of among others Syncerus caffer in Lascaux (15,000 years old; southern-France) and Altimira (13,500 years old; northern-Spain) are indicative for an internalized ‘system of thought’ supporting the notion that this large herbivore of the African savannah was an important prey animal for early hominids.
Keywords:
Homo sapiens, human brain, neocortex, encephalization, obese mouse model, C57bl6, encephalization, human evolution, ecology, savannah, Syncerus caffer, bipedalism, Out of Africa, Systems Biology, lipidomics.
Introduction
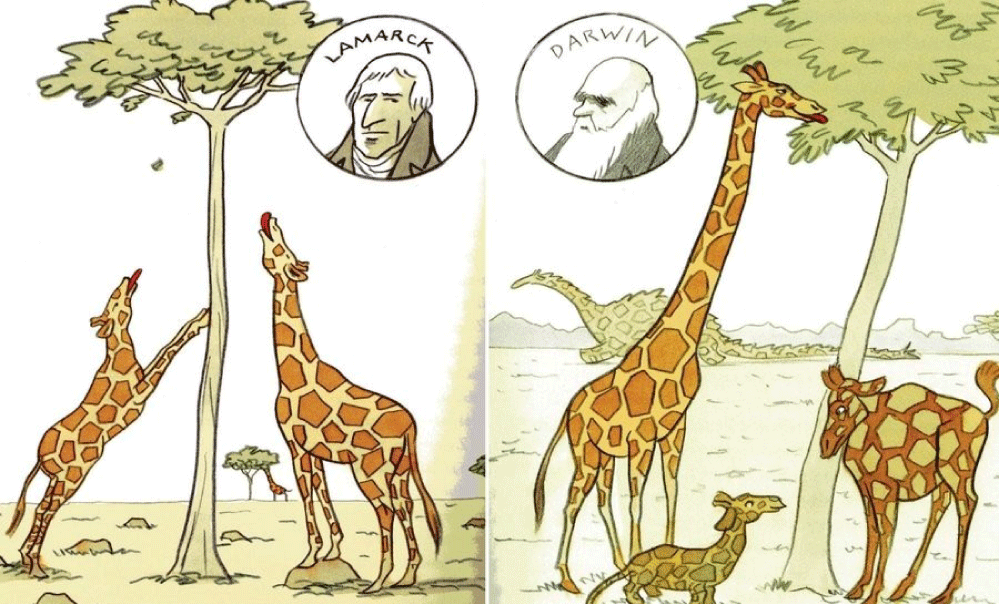
This review has a pretty ‘loaded’ title immediately criticizing Charles Darwin’s all-inclusive evolution theory of negligence. But the reality is that Darwin did not have the modern laboratory facilities scientists of the 21st century have at their disposal. Figure 1 presents the theories of the two leading scientists of evolutionary biology of the 18th century, Baptiste Lamarck and Charles Darwin in one drawing [1,2].
Figure 1: Lamarckism, a theory of evolution is also called the inheritance of acquired characteristics or soft inheritance. It is inaccurately named after the French biologist Jean-Baptiste Lamarck (1744–1829) and based on the principle that physical changes in organisms during their lifetime—such as increased development of an organ or a body-part due to increased use—could be transmitted to their offspring (1. Lamarck 1809). Darwinism is a theory of biological evolution developed by the English naturalist Charles Darwin (1809–1882) and others, stating that all species of organisms arise and develop through the natural selection of small, inherited variations that increase the individual’s ability to compete, survive, and reproduce (2. Darwin 1859).
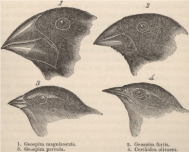
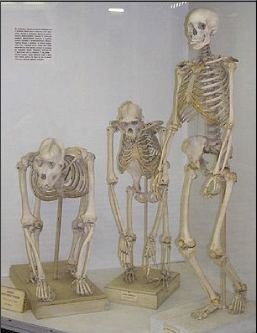
Darwin accepted and described over 150 years ago in his masterwork “On the Origin of Species (by Natural Selection)” the evolutionary laws of the “Struggle for Survival” and the “Survival of the Fittest”. Evolutionary laws aimed at improving a species and to adapt it to environmental factors which can be harsh and eliminate the “weak” unadjusted individual from a population. The environmental trigger for this elimination was often the supply of food. Darwin also described how within such a population there was sufficient time to recover with better adapted species. His main support for this initial hypothesis came from the finches he had collected on the various Galapagos islands. He caught several finches with different beak shapes (Figure 2A) on the different islands of the Galapagos Archipelago and his perception was that they came from a common ancestor. This formed the basis for his “Tree of Life (TOL)”, from which he carefully formulated his initial hypothesis as shown in Figure 2B [3].
Figure 2A: Figure 2A: Charles Darwin caught several finches with different beak shapes on the different islands of the Galapagos Archipelago and his perception was that they came from a common ancestor. This formed the basis for his “Tree of Life (TOL)”, from which he carefully formulated his initial hypothesis as shown in Figure 2B
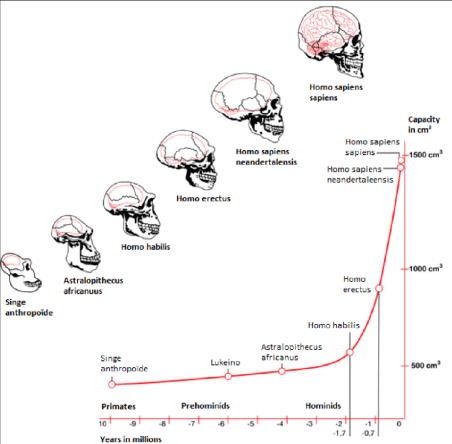
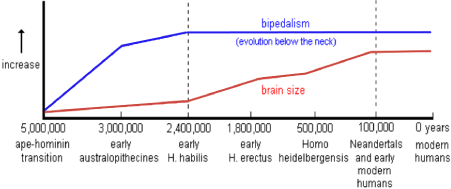
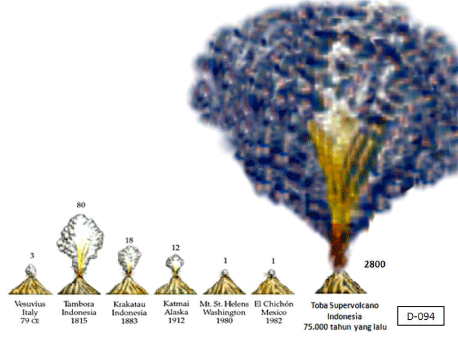
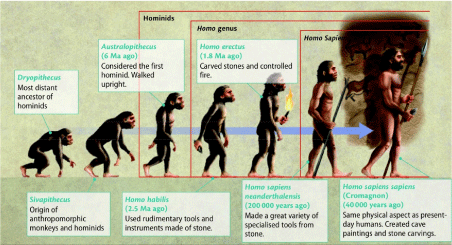
Unfortunately, Darwin did not accept the scientific challenge of explaining the overgrown human neocortex in his book “On the Origin of Species by Natural Selection”. Recent fascinating discoveries in several research fields provide a blending of ecological-, anatomical-, physiological-, biochemical-, (bio)medical and nutritional data (‘the Modern Synthesis of Understanding’ á la Sir Julian Sorell Huxley 1187-1975). Their interconversions and their evolving conclusions bring us closer to a single innovative and comprehensive evolutionary theory about human brain growth or encephalization as a replenishment to Darwin’s 1). “On the Origin of Species by Natural Selection” (1859) or its successor 2). “On the Descent of Man” (1871) in both of which Charles Darwin neglected to give a plausible explanation for the tremendous growth of the human brain over the last 75,000 years (Figure 3) [4].
In chapter 14, ‘Recapitulation and Conclusions’, Darwin only wrote: “In the distant future I see open fields for far more researches. Psychology will be based on a new foundation, that of the necessary acquirements of each mental power and capacity by gradation. Light will be thrown on the origin of man and his history”.
One of the prominent ways to follow the evolution of the human brain is through direct evidence in the form of fossils. The evolutionary history of the human brain primarily shows a gradual increase of brain size in proportion to body size during the development from early primates to hominids and ultimately to Homo sapiens. Because fossilized brain tissue is rare, a more reliable approach is to observe anatomical characteristics of the skull which provide insight into brain characteristics. One such method is observing the endocranial cast (also called endocasts). Endocasts occur when, during the fossilization process, the brain deteriorates, leaving a space that is filled up by excessive sedimentary material. These casts give an impression of the lining of the brain cavity, making it possible to visualize what was there [5]. This approach, however, is limited as to what information can be collected mainly providing information about the size of the brain (skull capacity or endocranial volume), prominent sulci and gyri, and the size of dominant lobes or brain regions [6]. Although endocasts are extremely useful in revealing superficial brain anatomy, they cannot reveal a brain structure, especially from deeper brain regions. By determining skull capacity in relation to the total number of neurons present in primates, fossil evidence allows for an estimation of the number of neurons [7]. Herculano-Houzel 2012). Despite the limitations to using endocasts, they can provide a basis for understanding human brain evolution primarily showing a gradually growing brain. This trend which has led to the current human brain size indicates a 2-3-fold increase in the last 3 million years [8]. Especially over the last 170,000 years, an excessive exponential brain growth has been observed from Homo habilis towards Homo sapiens (Figure 3). Few things demonstrate the distinctive character of research and the various research schools, such as research into human evolution. On the one hand we have research with a long tradition in human evolution - already suggested by 1.Lamarck (1809) and 2.Darwin (1871), that human ancestors descended from the trees and moved to the open savannah - firmly based on the ‘Savanna Dry-Land Hypothesis’ (SDLH), which explains most human evolutionary traits, such as walking on two legs (≈bipedalism).
The SDLH first came to prominence, however, with the discovery of Australopithecus africanus by Raymond Dart in 1924. In an article on the discovery, published in the journal Nature, Dart wrote:
“For the production of man a different apprenticeship was needed to sharpen the wits and quicken the higher manifestations of intellect – a more open veldt country where competition was keener between swiftness and stealth, and where adroitness of thinking and movement played a preponderating role in the preservation of the species. Darwin has said, “no country in the world abounds in a greater degree with dangerous beasts than Southern Africa.” and, in my opinion, Southern Africa, by providing a vast open country with occasional wooded belts and a relative scarcity of water, together with a fierce and bitter mammalian competition, furnished a laboratory such as was essential to this penultimate phase of human evolution” [9,10].
In the latter parts of the 20th century, new fossil evidence began to emerge which called the SDLH into question. These newly discovered remains showed indications that human ancestors were still well adapted to climbing trees, even after they had begun to walk upright [11].
Not everyone was willing to write off the savannah hypothesis. A poor definition of what a savannah actually is, contributed to this. Critics of the hypothesis often saw the savannah as open grasslands with sporadic tree growth. However, savannas can have a high tree density and can also be humid. The large difference between savannas and forests is the lack of grasses in the latter. Cerling et al (2011) developed a method to determine the forest cover of ancient landscapes, thus no longer requiring a definition of what a savannah is. By distinguishing between the C3 plants of the tropical forests and the mix of trees and C4 grasses of the savannah, they investigated the stable carbon isotope content of paleo-sols from some sites in East Africa. They described landscapes varying between forest, woodland/bushland/shrubland, wooded grasslands and grasslands. They concluded that the early hominids lived in a more open environment than the hominin Australopithecus, rendering the savannah hypothesis still a plausible possibility [12].
Using a similar argument - still at an ecological perception. Domínguez-Rodrigo (2014) stated that the usual division of landscapes into grassy, wooded and wooded is of little use, because it tells nothing about the evolutionary pressure on mammals. For example, the selection pressure of grass fields in tropical forests is incomparable to the grasslands of savannas. Tropical forests harbour many different species of trees, while savannas only have a few species, which hardly carry any fruit [13].
Another factor is that of scale. Paleoanthropologists often investigate only the site itself, an area of several hundred to thousands of meters. These habitats are referred to as ‘biomes’ (Figure 4), but in fact a proper ecological definition would require an area of many hundreds of kilometres in order to be able to elucidate until now unknown social lifestyle patterns such as hunting or foraging as hunter gatherers over a certain area, which yields sufficient food [14] (Figure 5).
Figure 4: ‘Biomes’ of the World with at the continent Africa the largest Savannah; a mixed woodland grassland ecosystem characterised by the trees being sufficiently widely spaced so that the canopy does not close. The open canopy allows sufficient light to reach the ground supporting an unbroken herbaceous layer consisting primarily of grasses (14. Paine et al 2019).
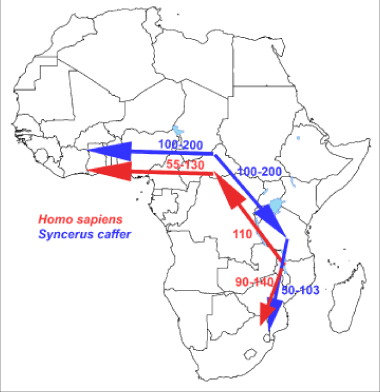
Figure 5: Similarity of migration routes of early hominids with those of the African buffalo (Syncerus caffer) –hunter-prey correlation- which most likely expanded and diverged in the late to middle Pleistocene from an ancestral population located around the current-day Central African Republic with the Cape buffalo undertaking successive colonization events from Eastern toward Western and Southern Africa (9.van Ginneken et al 2017).
Modern human expansion from Africa has important implications for understanding the genetic and phenotypic structure of extant populations. Most of the diverse bovid species occurred in Africa. Their maximum concentration was reached in the savannas of eastern Africa possibly in the period in which the Cape buffalo evolved as a separate subspecies, according to the net sequence divergence compared with other subspecies (Figure 6). These two observations agree with the hypothesis of a rapid evolution of Cape buffalo based on fossil data [15]. Additionally,
The African buffalo (Syncerus caffer) exhibits extreme morphological variability. Recent molecular analysis using a comprehensive set of mitochondrial D-loop sequences from across the entire range of the species converged on the existence of two distinct lineages, corresponding to a group encompassing West- and Central-African populations and a group encompassing East- and Southern-African populations. The two lineages of the African buffalo most likely expanded and diverged in late to middle Pleistocene with strong indications for a population expansion in both lineages which diverged between 145,000 to 449,000 years ago [17]. Arguing thus, the study identified the most probable historical migration routes, based on a Bayesian analysis, with the Cape buffalo undertaking successive colonization events from Eastern toward Southern Africa (Figure 5). Furthermore, their analyses indicate that, in the West- Central African lineage, the forest eco-phenotype may be a derived form of the savanna eco-phenotype and not vice versa, as has previously been proposed. This recent study supports earlier paleontological findings on bovid fossils from Elandsfontein, located in the south-western Cape Province, South Africa, which comprise 7,257 individually numbered specimens from 18 buffalo species. Taxonomic comparisons with Olduvai Gorge and other African sites and the high percentage of extinct forms imply that the bones accumulated in the earlier part of the Middle Quaternary, probably sometime between 700,000 and 400,000 years ago data. Also based on analysis of mitochondrial and Y-chromosomal loci from these ancestors of the modern African buffalo, it was concluded that it had a Pleistocene origin and population expansion [18]. In conjunction with data of the paleontological studies, at the coast of South Africa at fossils of ancestors of the present modern African buffalo. Similarity of the migration routes of early hominids and these early African buffalo species supports the “Out-Of-Africa” theory. We hope that our results and our initial evolutionary hypothesis termed “The African Savannah Buffalo” hypothesis will stimulate further work on this important topic. The major outcomes of our “African Savannah Buffalo hypothesis” (“ASB-hypothesis”) are depicted in Figure 5 which gives the similarity of dispersal/migration routes of early bovines and early hominids (direct hunter-prey correlation/association).
Explanations for brain evolution in primates must be seen against the background of the challenges associated with the evolution of coordinated, coherent, connected social groups which require new social behavior for their solution, along with the specialized cognition and neural substrates that support it. Here again it has been questioned if the Savannah Hypothesis Model presents an ecological model providing sufficient ‘substrate’ (≈ ‘meat’ see further) with a biochemical composition meeting the requirements of the developing human brain in terms of omega-3 & omega-6 PUFAs. So, here raise again a crucial but frequently overlooked issue, i.e., the fact that the evolution of large brains mainly requires energetic constraints which must be overcome [19]. To meet this requirement, the herds of the ruminant and large herbivores of the African savannah, the African buffalo (Syncerus caffer), provided a tremendous amount of biomass (meat) to hunt for. The African forest buffalo is a smaller variety of the African buffalo. The Cape buffalo weighs anywhere from 400 to 800 kg (880–1760 lbs), whereas the African forest buffalos are much lighter, weighing between 250 and 320 kg (550– 705 lbs). The African forest buffalo -foraging in the tropical forest of Nigeria lives in relatively small herds, as small as 3 and rarely over 30 compared to the well-studied Cape buffalo which occurs in herds of over 1,000 members [20]. According to Domínguez-Rodrigo (2014), the savannah (dry-land) hypothesis (SDLH) can still provide a good explanation for large human brains although the transition of environment has probably been less abrupt than some earlier authors thought. Observation of “brain steatosis” occurring in a C57bl6 obese mouse model raised for 40 days on a High-fat diet based on 24.0% bovine lard (16. van Ginneken et al 2017), a landmark discovery, directed us to the tremendous herds of the ancestors of the ruminant African buffalo (Figure 6), and the to the possibility that their products (meat & lard) where of such a biochemical composition, that they sustained the growth spurt of the human brain in late Pleistocene (van Ginneken 2020 in preparation).
In the last parts of the 20th century, new fossil evidence emerged which questioned the savannah hypothesis. The newly discovered ‘exotic’ Australopithecus ramidus -later called Ardipithecus ramidus- appeared to be half a million years older than the previously known A. afarensis and to have had a more monkey-like appearance [21]. After extensive research, a series of eleven articles published in the Science in 2009 concluded that Ar. ramidus preferred more wooded areas instead of the open grassland which would not support the climate-driven savannah hypothesis [22]. Fossils provided evidence that they were still well adapted to climbing trees even after they had started to walk upright [23]. The absence of cranial remains of Australopithecus species older than 3.5 million years has limited our understanding of the evolutionary history of this genus. In a recent article in Nature, Haile-Selassie and coworkers [24] describe a nearly complete hominin cranium of an Australopithecus species, dated to approximately 3.8 million years (Myr) ago, which fills a crucial gap in the hominin fossil record. The specimen (coded: MRD) showed a morphology that was more primitive than that of any previously known Australopithecus cranium, including features that link early Australopithecus to the Mio-Pliocene genera Sahelanthropus and Ardipithecus. This excavated fossil from a first hominin shows how complex it is to draw important conclusions about the human family tree (TOL) based on accidental excavations of a small number of rare fossils.
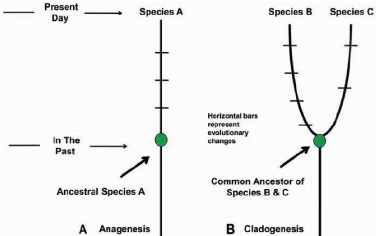
The major conclusion of Haile-Selassie and co-workers from this recent fossil discovery is that MRD as well as other discoveries from Woranso-Mille do not distort the proposed ancestor-descent relationship between A. anamensis and A. afarensis. The MRD cranium findings might also indicate that A. afarensis did not evolve from a single ancestral population. Most importantly, however, this 3.8 Myr nearly complete cranium founding of an early hominin shows that despite the generally accepted hypothesis of anagenesis (Figure 7) - A. afarensis did not appear due to phyletic transformation. It shows that at least two related hominins must have existed side by side in eastern Africa around 3.8 Myr ago, a finding which furthermore gave supportive evidence for a middle Pliocene humankind specimen of these Australopithecus hominins [24].
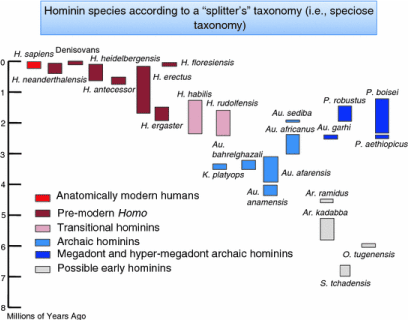
These discoveries have contributed to our understanding of human evolution by pushing the hominin fossil record in the Miocene era, and by possible taxonomic diversity, wider geograpHical distributions, the presence of multiple forms of bipedalism and a large adaptive shift associated with the origin of the genus Australopithecus. At the same time, these discoveries raise important questions about human taxonomy and systematics. Although most questions arise from the fragmentary (mostly dentognatic ≈ teeth) nature of the fossil record and the small sample size, some problems relate to the absence of fossils and skeletal elements that are informative in the system from critical time periods (Figure 8). But most important for the African savannah hypothesis is the assumption that these Australopithecus hominins obtained the bulk of dietary calories from African savannah plants.
Figure 8: Overview of the fossil record over a time frame of 8 million years. Just over the last 2-3 million years, the fossil record shows a more rapid increase of the cranial volume. While the cranium fossils of Australopithecus, Sahelanthropus and Ardipithecus were rare (single specimen) the fossil record increased tremendously over the time frame of the last 2 million years.
The savannah hypothesis plays a prominent role in this review manuscript and in the formulation of our initial hypothesis related to human brain encephalization. In this respect, mitochondrial DNA studies were extremely important providing supportive evidence that as proposed by Gonder [25], a large and diverse human population has persisted in eastern Africa which may be the cradle of humanity [26]. Genetic studies and fossil evidence indicate that archaic humans evolved to anatomically modern humans solely in Africa between 200,000 and 60,000 years ago [27]. In addition, members of one branch of Homo sapiens left Africa at some point between 125,000 and 60,000 years ago, and over time these humans replaced other populations of the genus Homo such as Neanderthals and Homo erectus [28]. The savannas of the world are currently undergoing another phase of change as modern expansion of the human population impinges on the fauna especially that of the early hominid species In this regard, the human adaptation began with Homo erectus who approximately 1.9 million years ago displayed strategies of life similar to Homo sapiens. The prominent change was the increased relative brain size that separated Homo erectus from Australopithecines [29].
We will in this review manuscript solely focus on the stage of exponential brain growth depicted in Figure 3 from Homo habilis (2.4 million to 1.4 million years ago with a skull capacity of around 550 cm3 towards the last 75,000 years when it was exponential from Homo erectus (averages of around 800-1,100 cm3 with a mean of around 950 cm3 for the African lineage; towards modern Homo sapiens (1500 cm3: averages about 1260 cm3 in men and 1130 cm3 in women, although there is considerable individual variation) [30,31].
In this respect, it must be remarked that the food resources produced by the African savannah ‘biomes’, are extremely important in our model of human brain encephalization. In earlier studies in a High-Fat Diet obesity induced C57BL6 mouse model on 24.0% bovine lard we observed accumulation of specific Triacylglycerols (TGs) under conditions of starvation [32] but also after exposure to a High-fat diet based on bovine lard, giving cause to the hypotheses that large amounts of TGs were the ‘prime movers’ in brain evolution for skull expansion (encephalization).
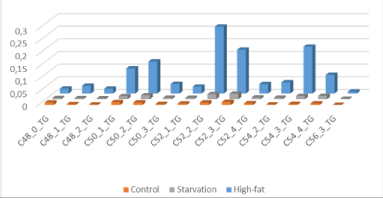
Thus, we hypothesized that the unique lipid composition of bovine lard (large amounts of unsaturated TGs C:50-1; C:50-2; C:52-2; C:52-3; C54-3; C:54-4 and C56- 3 TGs) might play a role in mammalian encephalization (Figure 9). In addition, we found a tight correlation of the HF-diet mouse brain composition with respect to these TGs to the HF-food diet (correlation coefficient r2=0.760 in comparison to control chow r2= 0.264).
Figure 9: “Brain steatosis” based on LCMS-data of whole mouse brain -“brain steatosis”- under two nutritional conditions: The specific molecular structure of bovine lard (high amounts of unsaturated C:50-1; C50-2; C:52-2; C:52-3; C54-3; C:54-4 and C56-3 Triacylglycerols (Source: 16. van Ginneken et al 2017).
Dietary quality has played a prominent role in theories of human evolution in general and the evolution of the human brain in particular [33,34]. Ideas of brain evolution centring on dietary quality have until present not been confined to humans and human evolution [35-37] coined the “Extractive Foraging Hypothesis” to explain the relationship in primates. They argued that a relatively large brain correlates with omnivorous feeding in primates which requires relatively complicated strategies for extracting high quality foodstuffs. The importance of a high-quality diet, and meat consumption in particular, has been a common theme [38]. One of the most memorable of these theories is known as the ‘Man the Hunter’ [39,40]. This theory argues that increasing amounts of meat in the hominid diet lead to increasing levels of cooperation among the males during the hunt, which lead to brain expansion and the associated development of cognition, language and symbolic culture. This hypothesis was fuelled by the realization that an increase in the apparent consumption of meat correlated with the increase in brain size seen in Homo habilis (cranium capacity of around 550 cm3) and Homo erectus (cranium capacity of around 800 – 1,100 cm3). It was also supported by the recognition in the archaeological record of the basic elements of a hunter-gatherer life-style (home bases and food sharing) [41]. Although the rather simplistic reasoning underlying the ‘Man the Hunter’ hypothesis has lost favour in more recent years [42], the importance of a high-quality diet, and meat eating in particular, have remained a common theme [43,44]. But what was the source of the meat? Luckily, the evolution, dispersal and speciation of the early African bovines – the ancestors of the African savannah buffalo – are rather well documented by three important studies: two mitochondrial DNA studies and one older paleontological study on fossils of early African bovines.
Not everyone was willing to write off the savannah hypothesis. As indicated above, a poor definition of what a savannah actually consisted of was essential to the debate. Critics of the hypothesis often saw the savannah as open grasslands with sporadic tree growth. African savannas, however, can have a high tree density and can also almost be the most productive ‘biomes’ of the world harbouring tremendous amounts of large mammalian herbivorous such as zebras, giraffes, elephants, ruminants like the African buffalo (Figure 10), small antelopes etcetera [45]. {Notify: the tropical forest is the most productive ‘biome’ [46]}.
The major difference between savannas and forests is the lack of grasses in the latter. According to Domínguez- Rodrigo, the savannah hypothesis can still provide a good explanation, although the transition from surroundings has probably been less abrupt than some previous authors thought.
Briefly, at the other extreme of the scientific paradigm, we have the ≈60 years old ‘Aquatic Ape Hypothesis’ (AAH) which states that our ancestors went through an aquatic phase [47] which led to the earlier mentioned “Aquatic Phase Hypothesis” (APH) evolutionary theory. The APH proposes that certain ancestors of modern humans were more aquatic than other great apes and even many modern humans, and, as such, were habitual waders, swimmers and divers. This is called the Hardy/Morgan hypothesis which argued that a branch of apes was forced by competition over terrestrial habitats to hunt for food such as shellfish on the sea shore and the sea bed leading to adaptations explaining distinctive characteristics of modern humans such as functional hairlessness and bipedalism [48].
Required biochemical model, based on the Savanah biomes for large human brains
The followers of the AAH & APH request a biochemical requirement for large human brains [49] which we will intensively outline in this review manuscript. It was argued by the AAH followers that brain components are dependent on food components such as Docosahexaenoic Acid (C22:6, ω-3; DHA) which is limiting because its synthesis from terrestrial plant food precursors from the savannah produces negligible amounts of this essential “fishy” Polyunsaturated Fatty Acid (PUFA) [50]. DHA is, however, abundant on the coastline where it is produced by microalgae and seaweeds which are consumed by fish and bivalves. DHA supports the development of very large marine mammalian brains in the seas [50-53].
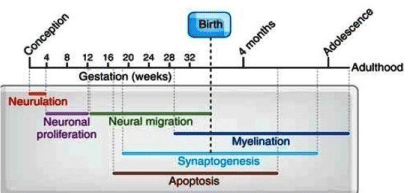
Evolutionary sciences, studying the issue of human brain growth (encephalization), focusing on embryology and fetal development of the brain during gestation, are quite informative (reviewed: 54). Modern human brains accumulate DHA for up to 18 years, most aggressively from about half the pregnancy to about two years old [52]. We recently reviewed the literature related to PUFA requirement during gestation because we aimed to give a comprehensive explanation for the molecular bases of “The Fetal Origin Hypothesis of Mental Disorders” FOHOMDhypothesis based on LC-MS studies at post-mortem Type-2 diabetes (T2DM) human brain for red and white matter supported by whole brain matter of a juvenile C57bl6 mouse model. This study clearly showed that during the first postnatal period months (Figure 11), during gestation a total amount of 600 g Essential Fatty Acids (EFAs) are transferred from mother to fetus during one term pregnancy [54,55].
Cortical white matter increases from childhood (~9 years) to adolescence (~14 years), most notably in the frontal and parietal cortices [56,57]. Cortical grey matter development peaks at ~12 years of age in the frontal and parietal cortices, and at 17 years in the temporal lobes (with the superior temporal cortex being last to mature) for women and reached full maturity at age 16-17. For men, full mature was reached at age 18. In terms of grey matter loss, the sensory and motor regions mature first, followed by other cortical regions. Human brain maturation continues to approximately 20 years of age [58].
In describing a proper initial hypothesis after this lengthy introduction, the evolutionary survey conducted by Tuomisto et al 2018 [60] is very useful providing an overview of all evolutionary traits involved in the choice between the Savannah dryland (SDL) hypothesis and the Aquatic Ape Hypothesis (AAH) [59,60]. The first hypothesis is in our case (proposing a biochemical model) based on the meat of the African buffalo (Syncerus caffer), which is the major source for essential fatty acids (EFAs), polyunsaturated fatty acids (PUFAs) and very long chain fatty acids (VLFA) providing the growing human brain during a course of evolution with the biochemical building blocks it needs (van Ginneken 2020 in press). The other theory, the AAH, presents an aquatic ecological explanation solely for the required PUFAs, which the growing human brain needs and which are provided by an aquatic environment with new food resources such as seaweeds, finfish and bivalves as evolutionary driving traits for human brain encephalization. No information related to the important VLFA for human brain encephalization which we observed earlier in a High Fat diet -based on bovine lard- induced obese C57bl6 mouse model with “overgrown” brain is incorporated in this “model”.
Table 1: The hypotheses on the evolutionary origin of human traits that were included in an online survey of 60.Tuomisto et al (2018) to find out how popular they are among scientists. If ambiguous, the ‘by one statement phrased’ hypotheses is followed by a letter depicting the trait: B=Bipedalism; E=Encephalization; F=subcutaneous fat; N=nakedness; L= descended larynx; S=speech; O=other (source: Table 1, 60.Tuomisto et al 2018; modified).
Bipedalism |
Big Brain |
Nakedness |
Subcutaneous. Fat |
Descended- Larynx |
Speech |
Other traits |
Energy efficiency |
Meat |
Skin contact baby |
Energy supply |
Articulation |
Larynx S |
Baby swimming |
Thin branches |
Fish |
Skin contact sex |
Thermoregulation buoyancy |
Sexual selection L |
Diving S |
Nose |
Wading |
Cooking |
Cleanliness |
Thermoregulation savanna F |
Diving L |
Bipedalism S |
Smell |
Thermoregu- |
Social organization E |
Ectoparasites |
Sexual selection F |
X |
Reassurance |
Webbing |
Better view |
Hunting E |
Drag-Thermo-regulation |
X |
X |
Social S |
Endocrine glands |
Foraging |
Language |
Overheating |
X |
X |
Hunting S |
Sweating |
Carrying food |
Warfare |
Body-size |
X |
X |
Culture |
Diving O |
Tool use |
Neoteny |
Clothes |
X |
X |
X |
Apnea |
Sexual selection B |
Bipedalism E |
X |
X |
X |
X |
Fond of water |
X |
Nakedness E |
X |
X |
X |
X |
|
From the survey of Tuomisto et al., [60] it becomes clear that the AAH is not very popular in the International Scientific Community (ISC). Half of the respondents of the survey fully or mostly agreed with the statement that the AAH, “Is not needed because all human traits (Table 1) can be explained by terrestrial scenarios”. In addition, the survey also indicated that professionals in the field of human evolution are more critical towards the AAH than outsiders.
The evolutionary human traits mentioned in Table 1 can briefly be interrelated by a scenario of evolution characterized by internal inherent drivers which emerged during the course of human evolution and which explains to a large extent the complexity of human evolution. It involves the following human evolutionary traits:
a). The large brain evolved because complex social organization required higher intelligence;
b). The subcutaneous fat layer evolved to serve as an energy reserve for the developing brain;
c). The feature of articulate speech evolved because there was social pressure for elaborate communication;
d). The larynx descended because this was required by articulate speech;
e). Bipedalism evolved to make the use of tools and weapons easier;
f). Nakedness evolved to avoid overheating during hunting.
Next, the evolutionary human traits mentioned in Table 1 - which are based on the results of the survey - are ranked in order of evolutionary importance. ‘Large Brains’ emerges as the most important human trait, which outcome emphasizes the importance of this review, where an explanation for human brain encephalization is being sought.
So, from all this information, we now must formulate a section which will lead to an evolutionary biochemical hypothesis describing the ecological origin of the molecular devices (EFAs & LCFA & PUFAs) which are the building blocks of the overgrown human brain. We hypothesize that these Fatty Acids (FAs) are delivered by the meat and lard of the (ancestors) of the African buffalo (Syncerus caffer) which co-inhabited the savannahs of East and South Africa (Figure 4 & 6), about 100,000 years ago with the ancestors of modern Homo sapiens. This evolutionary model must explain the exponential growth spurt and doubling of the human brain volume from Homo habilis (cranium capacity of around 550 cm3) towards modern Homo erectus (cranium capacity of around 800-1,100 cm3) over a time-frame of around 1 million years ago (Figure 3) and the growth spurt over the last 70,000 year towards Homo sapiens (cranium capacity of around 1,400-1,500 cm3) (Figure 3).
Human evolution is a blending of Paleontology Anthropology, Psychology, Physiology and Anatomy and finally Biochemistry: “the Modern Synthesis of Understanding”; Huxley 1187-1975)
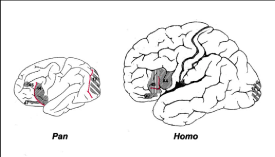
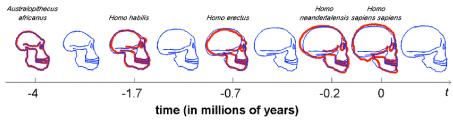
Human evolution is the evolutionary process that led to the emergence of anatomically modern Homo sapiens (61). In Figure 12, a reflection is depicted of human evolution from its first separation of the apes -the chimpanzee lineage (Pan)- towards the last common ancestor of archaic Homo sapiens [61]. Figure 13 shows the related evolution of the brain volume. The human and great ape lineages diverged approximately 5-6 million years ago (Figure 12). (Striedter 2005 [62]) mainly based on anatomical and morphological characteristics related to a hand grip. It is the human genus that dominates the areas of making and using more complex tools [62]. Metacarpal styloid process enables the hand bone to lock into the wrist bones, allowing for greater amounts of pressure to be applied to the wrist and hand from a grasping thumb and fingers. This third evolutionary trait is particularly characteristic for the Homo-lineage and may indicate an anatomical and morphological commitment to tool-related manipulated behaviors, in contrast to the Pan-lineage which did not develop these anatomical changes to the hand [63]. It allowed humans the dexterity and strength to make and use complex tools. This unique anatomical feature separated humans from apes and other non-human primates and is not seen in human fossils older than 1.8 million years [64].
Figure 12: Reflection of the split between the ancient ancestors of the chimpanzee lineage (Pan-lineage) with its brain volume of around 410 cm3 towards the lineage of modern man Homo sapiens (Homo-lineage) with a brain of around 1340 cm3 (≈1500 cm3), about 3 times the volume of a chimpanzee during course of evolution over 5-6 million years. According to various researchers, the evolutionary characteristic ‘upright walking’ originated relatively early in the evolution - about 2.4 million years ago - while the characteristic large, round 1500 cm3 brain of Homo sapiens originated some 100,000 years ago (62.Striedter 2005; 61.McHenry 2009).
Evolutionary plausible biochemical model for vertical walking of early Hominins and Hominids resulting in hypoxia and brain growth (encephalization)
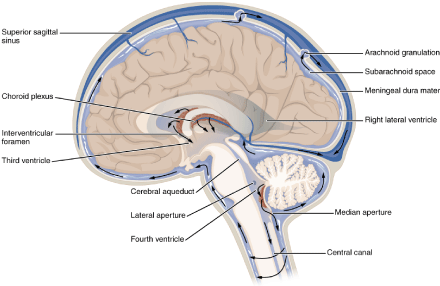
In this review, I will also propose an evolutionary plausible biochemical model explaining vertical walking by early Hominids based on optional hypoxic brain conditions. The biochemical consequences of this mechanism which we will describe in this paragraph, solely serving maintenance of the redox potential via a reversal of the β-oxidation, results in White Adipose Tissue (WAT) formation in the human brain or in other words, evolutionary “encephalization”. Except for adipose tissue, the human brain contains the highest concentration of lipids. Sixty percent of the brain structural material (dry weight) consists of lipid [65,66]. Due to its low vascularization degree it has been demonstrated that in the brain, low oxygen conditions (hypoxia) might occur. We postulate that hypoxia of the “fatty” human brain results in fatty chain elongation and consequently in human brain growth. The “fatty” human brain has been shown via neurosurgery to become hypoxic at greater depth [67]. In order to maintain the redox balance and keep the Krebs cycle spinning, we proposed a biochemical model of “reversed β oxidation” (fatty chain elongation) also leading to fat synthesis and brain growth. Literature data support our biochemical model showing that periods of intermittent hypoxia stimulate brain growth via nonrespiratory neuron restoration [68]. In conjunction with the suggested biochemical model, we propose that the human brain is still growing, which quite relevant from an evolutionary point of view. One of our major goals was to try to unravel the function of the cerebrospinal fluid (CSF) in relation to brain growth. In this respect, three main functions are presently recognized: i). CSF protects brain and spinal cord from trauma; ii). CSF supplies nutrients to nervous system tissue; iii). CSF removes waste products from cerebral metabolism [69].
Cerebrospinal fluid (CSF) is a clear, colorless body fluid in the brain and spinal cord. It acts as a buffer or buffer for the brain and offers mechanical and immunological protection to the brain in the skull. CSF also serves a vital function in cerebral autoregulation of blood flow in the brain (Figure 14) [70]. In studying human evolution – notably the unexplained encephalization - some interesting observations have recently been made regarding a
Figure 15: Based on fossil evidence, the evolutionary driving forces changed the body position from quadrupedal (Qp) way of moving or four-legged animals (on the left) like its ancestor from early humanoids to vertical walking of modern human beings (Homo sapiens, on the right). Our hypothesis - based on pH values of the brain fluid - is indicative of a wide range of lactic acid values providing a fundamentally new insight into brain energy metabolism by showing that completely oxidized glucose can be exported as lactate via glymphatic-lymphatic fluid transport. MRI and PET have the disadvantage that they can neither measure lactic acid directly in the brain in vivo, nor in circumstances of heavy exertion.
This is important information as we can follow two approaches when studying the question of human evolutionary encephalization: i). Enlightening the path of human evolution can mainly be based on fossil specimens. ii). The other route is that we believe that human evolution is literally engraved in our body and as such we can reconstruct human evolution based on modern biochemical, physiological and biomedical findings. Fossil and molecular evidence suggests that the earliest ancestors of the human family lived in wooded areas in equatorial Africa in the late Miocene era about 8 to 10 million years ago. In this context, another theory considered the efficiency of walking upright (Figure 15), suggesting that hominids evolved to walk upright in response to climate change [73]. This would support our hunter-prey correlation for early humans as early hunters began walking on two legs due to evolutionary driving forces such as the bounty of the hunting.
Using a comparative physiological approach, it has been hypothesized that the CSF system is primarily developed to preserve the chemical environment, including the neuroendocrine pathways, necessary for the function of the cells of the central nervous system [74]. In this manuscript, we attempt to describe some of these issues of human evolution in order to unravel the function of Cerebrospinal Brain Fluid (CSF) in relation to brain development. The human brain has an extremely high oxygen consumption which is relatively constant over time. In the average adult human, despite constituting only 2-3% of the total body weight, the brain receives 15% of the heart minute volume, and consumes ~ 20% of the body’s own oxygen and 15% of the total body veil [75]. This high level of metabolism is remarkably constant, despite strongly varying mental and motor activity and is remarkably high compared to other organs or tissues [76]. Until recently, it was generally recognized that the human brain cannot cope with states of anaerobioses (low or no oxygen conditions) [77]. However, when human brain pO2 was measured by pseudoneurosurgery using brain-inserted probes performed in 27 patients, the mean pO2 was shown to decrease with a brain depth that reached a hypoxic level of 23.8 ± 8.1 mmHg at 22 to 27 mm below the dura. Scholars agree that if the pO2 of the brain tissue increases to over 35 mmHg (4.6%), normal oxygenation of the brain tissue must be ensured. In addition, photoacoustic tomography (PAT) of blood oxygenation of the human brain during a bout of exercise showed that the human brain became hypoxic [78].
Since the beginning of the 1970s, more supporting evidence emerged for increased glycolysis and lactate release from the brain into the blood during brain activation in normal subjects with low plasma glucose levels during normal and physiological pathological conditions. Heavy exercise increases lactate levels in the blood, which gave cause to the assumption that lactic acid could be an alternative substrate that is oxidized in increased amounts in the exercised brain [79]. These observations were only made in laboratory studies on cultured cells and brain disks, but not in the in vivo brain which is currently impossible using proton 1-NMR spectroscopy due to the presence of overwhelming water (and sometimes lipid) signals [80] or using a PET scan [81]. A conventional PET imaging technique employing 11-C-glucose only detects the presence of radioactivity and not the type of carrier. Therefore, it cannot distinguish between the native radiotracer 11C-glucose and its metabolites pyruvate and lactate [82]. Moreover, in these devices it is only possible to measure under sedentary conditions, so that currently lactic acid data of whole brains cannot be obtained under severe exercise conditions (walking or running). That is why we chose to use a more ‘traditional’ approach using cerebrospinal fluid pH values in autopsies of 292 test subjects of the “Netherlands Brain Bank”, a brain material collection initiated in 1987. In accordance with the revised observations of increasing evidence suggests that glucose is not completely oxidized, but can be exported as lactate via glymphatic-lymphatic fluid transport for refueling the brain. Lactate is generated and oxidized by neurons and astrocytes, but the size and direction of cellcell lactate shuttles linked to the oxidation or release from the brain has yet to be determined in vivo. Continuous release of lactate into the human brain is suggested for exercising somatic tissue (for example, walking or running on two legs) [83]. When studying metabolism in relation to brain functioning - in a horizontal position - in relation to hypoxic conditions and lactic acid production, one can ask three important questions: 1). Through which biochemical brain energy metabolism route is sufficient energy (ATP) produced to provide the hypoxic brain cells with sufficient ATP and what are the consequences of this derailed hypoxic energy metabolism? 2). Under aerobic conditions, the driving force of oxidative phosphorylation is the electron transfer potential of NADH or FADH2 relative to that of O2. So, how is the redox balance maintained in these brain cells under these hypoxic conditions? 3). Via which biochemical routes are the metabolites obtained for the production of new cells in the growing brain?
These three issues will be intertwined in the following sessions of this review manuscript. Next, we will present a biochemical model - possibly involved in anapleurotic reactions - to keep the Krebs cycle spinning for macromolecule precursor production essential for further brain cell growth and proliferation.
The purpose of understanding cellular and subcellular contributions during brain activation is a long-standing relevant unresolved issue that is crucial to understanding the energy metabolism of the entire human brain, which in turn is central to understanding interactions between astrocytes and neurons. It is unmistakable that the MRI material of sedentary imaging research in the in vivo human brain made enormous progress, but that the demand for lactic acid as a substrate for nourishing the brain by cells during increased metabolism followed by recovery still needs to be established. Under normoxic sedentary conditions, glucose is the mandatory fuel for adult brains, but lactate produced from glucose by astrocytes in brains during activation has been suggested to serve as a neuronal fuel [84]. However, stoichiometric metabolic requirements for substantial lactate shuttling and oxidation were not met in this model, and therefore our observations of a scattered cerebrospinal fluid pH can largely supplement the gaps in explaining how to feed the brain under different exercise conditions. Insight into preferential upregulation of glucose compared to oxygen use due to e.g., increased activity is a central theme for clarifying brain energetics. Our most important findings were as follows. First, in postmortal autopsies of the “Dutch Brain Bank” we determined the pH of the cerebrospinal brain fluid (CSF), which appeared to be almost one pH unit lower 6.53 ± 0.315 (n = 291) than normal extracellular cells. liquid of 7.4 ± 0.1[85]. This is an important observation indicating that lactic acid can be the culprit in the hypoxic metabolism of human brains exhibiting oscillating patterns. Moreover, the study (35) with “Netherlands Brain-bank” material can be important to determine the role of lactic acid as fuel and redox potential in the entire human brain. Patients died under all kinds of conditions - not just hospitalized under cachexia - and this explained the enormous variation and dispersed pattern of the pH of the cerebrospinal fluid as an indirect indicator of lactic acid. The human brain is a strong oxidative organ, but during activation the glycolytic flux is preferably regulated up, even if the oxygen supply is sufficient. The biochemical and cellular basis of metabolic changes during brain activation and the fate of lactate produced in the brain are important yet unsolved problems central to understanding brain function [86].
In addition, numerous MRI examinations have indicated that there is an elegant link between energy demand and brain activity (for example emotions associated with blood flow). For intense practice outside the MRI, this remains an unexplored area while it still needs to be resolved which energy demand and metabolic mechanisms of the human brain respond both in their intensity and in their moment-to-moment dynamics to intensive exercise. In this regard, the “Astrocyte-Neuron Lactate Shuttle hypothesis model” suggested a temporal link between initial glycolysis in astrocytes and successive oxidative metabolism in neurons. However, the ultimate evidence for stoichiometric redox balance maintenance in combination with activitydependent fluctuations of the coenzyme nicotinamide adenine dinucleotide (NADH) could not be demonstrated [87]. Direct experimental evidence for this idea is still lacking, although in the next section of this manuscript, we will describe and emphasize our thoughts and assumptions on how the redox balance is maintained in the brain cell.
The shift from oxidative catabolism (energy production) to reductive anabolism (biomass synthesis), or from anabolism to catabolism by redox pairs, appears to be controlled by the vitamins niacin (NAD + ≈ nicotinamide adenine dinucleotide) and riboflavin (FAD ≈ flavin adenine nucleotide) which both play the role of coenzymes. These bio-couples are part of reductive and oxidative redox pairs / couples, such as nicotinamide adenine dinucleotide (NAD+ / NADH), nicotinamide niacin adenine dinucleotide phosphate (NADP+ / NADPH) and for riboflavin ≈ Vitamin B2 (FAD / FADH2) in combination with the universal energy carrier, adenosine triphosphate (ATP), the transmembrane potential (TMP) and finally the intracellular pH (pHi) and phosphorylation potential determined by in vivo 31P NMR [88]. The dynamics of these internal biological rhythms appears to oscillate when eukaryotic cells enter proliferation [89]. These highly ordered and wellorchestrated oscillations ensure genome duplication and cell membrane synthesis prior to cell division [90]. Similar oscillations related to refueling whole brains can also be expected in humans during metabolic activity that fluctuates between Basal Metabolic Rate (BMR), Routine Metabolic Rate (RMR) and Maximal Metabolic Rate (MMR), but still needs to be detected outside the MRI or PET scan.
Secondly, a most significant observation concerns the scattered pH concentration of the brain fluid, for both men and women, plotted against the brain weight. Three main functions are recognized in this context: i). CSF protects brain and spinal cord against trauma; ii). CSF supplies nutrients to nervous system tissue; iii). CSF removes waste products from brain metabolism, the so-called glymphatic system [91,92]. Previously, we hypothesized that evolutionary bipedalism required remarkable adjustments to hypoxic states in the brain, so that brain activity is not negatively affected by the horizontal position of the spinal cord. Experimental design was optimized for the absorption of lactate and glucose by the human brain and oxygen consumption during gradual training and recovery after maximal effort by measuring the arterial-internal jugular venous concentration differences (av differences) of six human volunteers [93]. Moreover, show that the glymphatic system is responsible for state-dependent changes in the concentration of brain lactate. Suppression of glymphatic function via i). Acetazolamide treatment; ii). Cisterna magna puncture; iii). Aquaporin 4 deletion or changes in body position, reduced the decrease in brain lactate which is normally observed when awake mice switch to sleep or anesthesia [94]. At the same time, the same manipulations reduced the accumulation of lactate in cervical but not in inguinal lymph nodes when mice were anesthetized.
Thus, from an evolutionary perception the study of [94] suggests that brain lactate is inversely correlated with glymphatic-lymphatic clearance which is dependent on a vertical position -walking on two legs- in comparison to quadrupedal (Qp) or four-legged animals like the ancestor of early hominids. This analysis provides fundamentally new insight into brain energy metabolism by demonstrating that glucose which is not fully oxidized can be exported as lactate via glymphatic-lymphatic fluid transport So, the transition from horizontal towards vertical has a long evolutionary transition time as the change from horizontal stance to vertical stance proceeded from generation to generation as corroborated by fossil evidence (Figure 15). Importantly, the gradual transition from vertical to horizontal kept pace with brain volume.
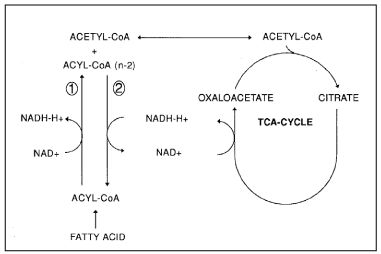
In this ‘whole human brain’ research manuscript, we consider the brain as a large fat particle or a “hub” or as a constellation of concentrated fat exposed due to its composition and low vascularization grade -but also due to strenuous exercise of somatic tissue- to hypoxic conditions. In order to conserve the energy from glucose as ATP, three major metabolic pathways are operative in the mitochondria: glycolysis (GLYC), the Krebs-cycle (KREBS) and β-oxidation (BOX). The KREBS and the BOX compete for the same redox couple NAD+/NADH under hypoxia. We stated earlier that in WAT (white adipose tissue), ischemic and/or hypoxic conditions may occur (95). Under these conditions, it can be questioned how the Krebs cycle persists in its activity and the redox balance is maintained. Earlier, we observed in a High-Fat diet obese C57bl6 mouse model with two relevant biomarkers for obesity and cardiovascular diseases (CVDs), based on product-to precursor ratios significant high increased with 304% elongase enzymatic activity (P‹0.0003***) [95]. So, High-Fat diet conditions lead to fat formation (≈white adipose tissue (WAT)) determined by elongase/desaturase enzymatic activity. Subsequently, we substantiated our assumption for a High-fat diet induced obese C57bl6 mouse model by biochemical model of reverse β oxidation” (r-BOX) unleashing the lipogenic potential to maintain the redox balance and keep the Krebs cycle spinning leading also to fat synthesis (Figure 16) and consequently to white adipose tissue (WAT) formation. In this way, the reaction is thermodynamically shifted in the direction of chain elongation which corresponds to fat synthesis [96]. Since fatty chain elongation consumes 2 moles of NADH in each cycle, this pathway provides a suitable mechanism to maintain the mitochondrial redox balance. So, this stochiometric scheduling -also for hypoxic whole braindemonstrates that the key constraint of r-BOX is redox imbalance.
Figure 16: Schematic representation of the redox coupling between the Krebs-cycle and fatty acid chain elongation. (1): Normal β-oxidation; (2): reversed β-oxidation. Under hypoxic conditions –which is the case in excessive WAT-tissue- both the Krebs-cycle and the β-oxidation need to maintain their redoxbalance which is accomplished on the one hand by performed lipid synthesis and on the other by “reverse β oxidation” (r-BOX) also leading to fat synthesis. So, a vicious circle is observed during severe obesity (95. van Ginneken et al 2016).
The extension of the fatty acid chain (lipid synthesis) during anoxia could indeed be stimulated in vitro by adding Krebs cycle intermediates such as glutamine [97] which is indicative of the redox coupling between Krebs cycle activity and fatty acid chain- elongation or “fat formation” (WAT) [9,99]. In the above sections, lipid metabolism has been proposed as a suitable mechanism to maintain redox balance in anoxic tolerant (in)vertebrate models by fatty acid (FA) chain extension (i.e. lipid synthesis) during anoxia / ischemia and to find its way to biomedicine [100]. This model, including the extension of the fat chain, ultimately leads to the formation of white adipose tissue ≈fat (WAT) and growth of the human brain or encephalization. In almost seven million years, the human brain has tripled in size, with most of its growth occurring in the last two million years. About 500,000 years ago, the average brain volume was 1,000 cubic centimeters and it continued to grow to around 1,500 cubic centimeters in today’s humans. The question remains whether the human brain is still growing. The adult human brain weighs on average about 1.2 - 1.4 kg, or about 2% of the total body weight (101. Parent & Carpenter 1995) with a volume of approximately 1260 cm3 in men and 1130 cm3 in women, although there is considerable individual variation. Van Ginneken (2019) [35 ]observed that the human brain is still growing in theory, and together with modern technology and computer science, this represents an area of unprecedented opportunities for humanity to further expand its civilization.
In summary, the biochemical model as depicted in Figure 16 plausibly explains continuous brain growth depending on the amount of brain tissue (mainly Triacylglycerols (TGs); van Ginneken 2020 in preparation), and the level of activity resulting in local brain hypoxic conditions as evolutionary trigger for early hominids and later the species Homo finally resulting in Homo sapiens.
From Homo habilis towards Homo erectus: division of Paleolithic times based on stone tools
The early Stone Age around 2.6 Myr (also known as the Lower Paleolithic) saw the development of the first stone tools by Homo habilis, one of the earliest members of the human family. These were basically stone cores with flakes removed from them to create a sharpened edge that could be used for cutting, chopping or scraping. These Oldowan tools -aged 2.6 million years- represent the first “mode” in the framework of tool technologies proposed by the British archaeologist Grahame Clark [101,102].
This marks the beginning of the Paleolithic, or Old Stone Age; its end is taken to be the end of the last Ice Age, around 10,000 years ago. The Paleolithic is subdivided into the Lower Paleolithic (Early Stone Age, ending around 350,000–300,000 years ago), the Middle Paleolithic (Middle Stone Age, until 50,000–30,000 years ago), and the Upper Paleolithic. The period from 700,000–300,000 years ago is also known as the Acheulean, when Homo ergaster (or Homo erectus) made large stone hand axes out of flint and quartzite, at first quite rough (Early Acheulian), later “retouched” by additional, more subtle strikes at the sides of the flakes. After 350,000 BP (Before Present) the more refined so-called Levallois technique was developed, a series of consecutive strikes, by which scrapers, slicers, needles, and flattened needles were made. Finally, after about 50,000 BP, even more refined and specialized flint tools were made by the Neanderthals and the immigrant Cro-Magnons (knives, blades, skimmers). During that time-period they also started to make tools out of bone. Mousterian-like tool industries were employed at that time also by early modern Homo sapiens in some areas of Africa and Southwest Asia.
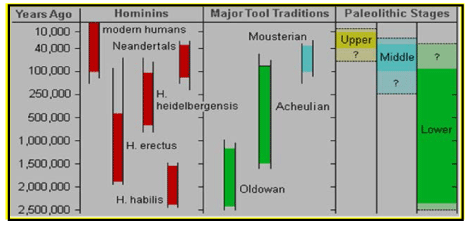
While until about 50,000-40,000 years ago the use of stone tools was common (figure 17), the transition to behavioral modernity seemed to have progressed stepwise. Each phase
Figure 17: Division of the Paleolithic Era and the corresponding evolving hominids which lived during that evolutionary time and used several kinds of tools, roughly divided in Oldowan tools (Lower Paleolithic stage), Acheulian tools (Lower- & Middle- Paleolithic stage) and Mousterian (Middle & presumably Paleolithic stage).
(Homo habilis, Homo ergaster -or Homo erectus-, Homo neanderthalensis) started at a higher level than the previous one, but after each phase started, further development was slow (103. Shulz et al 2002). Currently, paleoanthropologists are debating whether these Homo species possessed some or many of the cultural and behavioral traits associated with modern humans such as language, complex symbolic thinking, technological creativity etcetera.
It seems that they were culturally conservative, while maintaining simple technologies and foraging patterns over very long periods. Around 50,000 BP, modern human culture began to evolve faster. The transition to modern behavior is characterized as a Eurasian “Great Leap Forward” (104. Bar-Yosef 2002) or as the “Upper Paleolithic Revolution”, (105. Nowell 2010), because of the sudden appearance of distinctive signs of modern behavior in the archaeological file. Some scholars consider the transition more gradual, with some functions already appearing about 200,000 years ago under archaic African Homo sapiens (106. Ambrose 2001; 107. d’ Errico & Stringer 2011). Modern people began to bury their dead with animal skins, to make clothing, to hunt with more advanced techniques (such as the use of traps or scare animals) and to participate in cave painting (see § 8 108. McBrearty & Brooks 2000). Among concrete examples of modern human behavior, anthropologists include specialization of tools, use of jewelry and images (such as cave drawings), organization of living space, rituals (for example funerals with serious gifts), specialized hunting techniques, exploration of less hospitable geograpHical areas and barter networks. Debate continues if a “revolution” led to modern people (“the Big Bang of human consciousness”), or that evolution was more gradual (108. McBrearty & Brooks 2000). Modern people began to bury their dead with animal skins, to make clothing, to hunt with more advanced techniques (such as the use of traps or scare animals) and to participate in cave painting [107,108]. Among concrete examples of modern human behavior, anthropologists include specialization of tools, use of jewelry and images (such as cave drawings), organization of living space, rituals (for example funerals with serious gifts), specialized hunting techniques, exploration of less hospitable geograpHical areas and barter networks. Debate continues if a “revolution” led to modern people (“the Big Bang of human consciousness”), or that evolution was more gradual.
How early Hominids further evolved
What happened to us, humans, after the early split with the chimpanzee lineage around 5-6 million years ago? The hominid lineage did not march in a straight line to Homo sapiens. Instead, the early hominid lineage gave rise to many other (now extinct) hominids. Examining the fossils, the artifacts, and even the DNA of these relatives has helped us understand how this complex hominid tree evolved, and how modern humans came to exist. In the early Pleistocene, 1.5–1 Ma, in Africa some populations of Homo habilis are thought to have evolved larger brains and made more elaborate stone tools; these differences and others are sufficient for anthropologists to classify them as a new species, Homo erectus [109]. During the next million years a process of encephalization began, and with the arrival of Homo erectus in the fossil record, cranial capacity had doubled to 850 cm3 [110]. Homo erectus and Homo ergaster were the first of the hominids to leave Africa, and these species spread through Africa, Asia, and Europe between 1.3 to 1.8 million years ago. The increase in human brain size is equivalent to every generation having an additional 125,000 neurons more than their parents. Here are some of the important events in human history, with approximate dates, which reflect the evidence currently available. The evidence on which scientific accounts of human evolution are based comes from many fields of natural science. The main sources of knowledge about the evolutionary process has traditionally been the fossil record, but since the development of genetics beginning in the 1970s, DNA analysis has come to occupy a place of comparable importance. The studies of ontogeny, phylogeny and especially evolutionary developmental biology of both vertebrates and invertebrates offer considerable insight into the evolution of all life, including how humans evolved. The specific study of the origin and life of humans is anthropology, particularly paleoanthropology which focuses on the study of human prehistory [111]. From above mentioned research areas it is commonly acknowledged that the hominoids are descendants of a common ancestor. Furthermore, human evolution is characterized by some morphological, developmental, physiological, and behavioral changes that have taken place since the split between the last common ancestor of humans and chimpanzees. The most significant of these adaptations are bipedalism, increased brain size, lengthened ontogeny (gestation and infancy), and decreased sexual dimorpHism. The relationship between these changes is the subject of ongoing debate [112]. Other important significant morphological changes included the evolution of a power and precision grip, a change first occurring in Homo erectus [113]. This enabled Homo erectus to use tools e.g. for hunting. The use of tools has been interpreted as a sign of intelligence, and it has been theorized that tool use may have stimulated certain aspects of human evolution, especially the continued expansion of the human brain by a daily high-quality diet.
Human evolution usually covers only the evolutionary history of primates, in particular the genus Homo, and the emergence of Homo sapiens as a distinct species of hominids (or “great apes”). The possibility of linking humans with earlier apes by descent became clear only after 1859 with the publication of Charles Darwin’s “On the Origin of Species by Natural Selection” in which Darwin argued for the idea of the evolution of new species from earlier ones. Darwin’s book did not address the question of human evolution, saying only that “Light will be thrown on the origin of man and his history”. The amount of brain mass exceeding that related to an animal’s body mass is called encephalization. Quantifying an animal’s encephalization has been argued to be directly related to that animal’s level of intelligence. Charles Darwin wrote in his book “The Descent of Man” in 1871 twelve years after his famous “On the Origin of Species” (1859) : “No one, I presume, doubts that the large proportion which the size of man’s brain bears to his body, compared to the same proportion in the gorilla or orang, is closely connected with his mental powers.
Bramble & Lieberman (2004) proposed that early Homo were scavengers that used stone tools to harvest meat off carcasses and to open bones. They also proposed that humans specialized in long-distance running to compete with other scavengers in reaching carcasses [114]. Again, it has been suggested that such an adaptation ensured a food supply that made large brains possible.
Thus, encephalization has been tied to an increasing emphasis on meat in the diet or to the development of cooking, [115] and it has been proposed that intelligence increased as a response to an increased necessity for solving social problems as human society became more complex. Evidence from the hominid fossil record implies that major changes in diet and relative brain metabolism occurred with the emergence of the genus Homo. Not much research has been done to understand the evolutionary history of social life. This is partly because social behavior does not fossilize, making it difficult to deduce changes in evolutionary time. However, based on Bayesian comparative methods for different primates from different phylogenetic groups, behavior can be analyzed over evolutionary times. Theoretical models suggest two possibilities. First, the socio-ecological model states that group patterns are driven by individual responses to resource availability. Under this ‘unstructured’ model, if grouping patterns are optional, transitions between all possible social states (and polymorpHic states within a species) should be equally likely. Secondly, it has been proposed that the social complexity of primates increases step by step from solitary animals via small groups to large socially complex groups. From this ‘increasing’ complexity model, we would predict that pair living was the earliest form of social group, followed by more complex group patterns [116].
In addition, among mammals, humans also developed an especially broad repertoire of social interactions and understanding, which is driven by their unique ability to communicate through spoken language [117]. The human species developed a much larger brain than that of other primates – typically 1,330 cm3 in modern humans, nearly triple the size of that of a chimpanzee or gorilla which is around 410 cm3. The pattern of encephalization started with Homo habilis, which at approximately 600 cm3 had a brain slightly larger than that of chimpanzees, and continued with Homo erectus (800–1,100 cm3), reaching a maximum in Neanderthals with an average size of (1,200–1,900 cm3), larger even than Homo sapiens. The pattern of human postnatal brain growth differs from that of other apes. Characteristic for Homo sapiens is a pattern of heterochrony which can be defined as any genetically controlled difference in the timing or duration of a developmental process in an organism compared to its ancestors or other organisms like the chimpanzee. Several heterochrony’s have been described in humans, relative to the chimpanzee. In chimpanzee foetuses, brain and head growth starts at about the same developmental stage and continues at a rate similar to that of humans, but growth stops soon after birth, whereas humans brain and head growth continues several years after birth [118].
This leads to changes in example gratia in a different development of the brain structure between new-born humans and adults of around 30 years old. However, the differences between the structure of human brains and those of other apes may be even more significant than differences in size [119]. The increase in volume over time has affected areas within the brain unequally – the temporal lobes, which contain centers for language processing, have increased disproportionately, as has the prefrontal cortex which has been related to complex decision-making and moderating social behavior..
The brain is a very expensive organ in metabolic terms. The use of tools has been interpreted as a sign of intelligence, and it has been theorized that tool use may have stimulated certain aspects of human evolution, especially the continued expansion of the human brain. Paleontology has yet to explain the expansion of this organ over millions of years -and especially over the last 70,000 yearsdespite being extremely demanding in terms of energy consumption. It seems until presently to be incapable of doing so, so it is now up to modern biochemistry to provide an adequate evolutionary explanation especially for the growth spurt of the last 75,000 years from Homo erectus towards Homo sapiens (van Ginneken 2020 in press). The brain of a modern human consumes about 13 watts (260 kilocalories per day), a fifth of the body’s total energy consumption [120-123]. Increased tool use would allow hunting for energy-rich meat products and would enable processing more energy-rich plant products. Researchers have suggested that early hominids were thus under evolutionary pressure to increase their capacity to create and use tools [124]. It is argued that humans (and other primates) could not have developed a relatively large brain without also adapting a high-quality diet that would have permitted a reduction in the relative size of the gastrointestinal tract. Dietary change is therefore viewed as a ‘prime releaser’ in brain evolution. It has been argued that a high-quality diet -like a nutritional resource like meatwould have been necessary for the evolution of a relatively large human brains [125].
One of the main sources on the African savannah of high-quality food resulting in brain growth could have been the meat of the African buffalo (Syncerus caffer), or their predecessors, who would have migrated in large herds on the African savannah. In the dry season to the tropical jungles of Nigeria (West Africa) and in the wet season over the savannah in the direction of South Africa (see Figure 5). As noted earlier, we have found an important indication that the first hominids must have hunted for Syncerus caffer because we a direct correlation between the migration routes of the first hominids (based on archaeological excavations), and those of the ancestors of the African buffalo (Syncerus caffer) could be established. An image simulation of how these first hominids attacked such an African buffalo with their weapons is depicted in Figure 18. From this we can conclude that not only the nutritious meat needed for brain growth through this African savannah food resource must have stimulated brain growth, but that attacking these large herbivores of the African savannah required a dynamic group structure of these first hominids possibly already with language and possibly even with social group hierarchy, in addition to incredible courage, hunter strategies, and group interaction. Figure 18 is a clear example of what hunting requires in terms of complex social skills, like communication via sound or even language, possible leadership and social hierarchy, creation of weapons like spear, bow and arrow. Next, a good physical shape including hormonal regulated coping strategies (catecholamine regulated ‘fight or flight’ or ‘sitting and waiting’) under these extreme conditions of hunting is required to survive these conditions at the limits of existence (‘survival of the fittest’). The hunt itself can possibly be considered as an evolutionary pressure and selection trait because solely these individuals with the best traits for hunting under these primitive conditions, i.e., the smartest, quickest and bravest and those with the ability to communicate in a proper way with other members of the group possess all evolutionary favorable traits on which selection took place by such evolutionary forces as the hunt for a dangerous animal like Syncerus caffer, which even attacks lions.
Based on the social model of further suggest that the group- living (gregarious) patterns of social organisation in early hominids provide the scaffold for distinctive human traits mainly expressed during the hunt example gratia at Syncerus caffer (Figure 18), including coalition formation, cooperative resource defence, social hierarchy, the development of speech and language and in the end large brains [126].
The change to such a high-quality diet, which involved an increased proportion of animal-based products, must have been one of the ‘prime movers’ in brain evolution.
In this context and based on the biochemistry of the human brain, we have earlier in this review discussed ‘the ecological factors’ most probably surrounding the evolution of the human brain. Apart from the growth of the brain other anatomical, physiological and morphological features changed during course of evolution like: a). an increased importance of vision rather than smell; b). a smaller gut; c). loss of body hair; d). evolution of sweat glands; e). a change in the shape of the dental arcade from being u-shaped to being parabolic; f). development of a chin (found in Homo sapiens alone); g). development of styloid processes; h). development of a descended larynx (Figure 19).
Genetic Bottleneck theory
The full title of Darwin’s famous work was “On the Origin of Species by Means of Natural Selection,”. Natural selection is not the only evolutionary mechanism. Coincidence by accident for a favorable trait is also very important. Mutations occur regularly in the genetic material, due to copying errors or under the influence of chemicals and radiation. In every human embryo the DNA contains around 100 mutations, sometimes even more. Some are harmful and cause the embryo to die, others lead to minute changes that are not harmful but also not useful [127]. Which of those mutations will spread is largely dependent on random processes such as genetic drift. The smaller a population, the more coincidence will play a role. An extreme example of how coincidence influences evolution is genetic “bottle necks” in which a large part of the population is killed and the genetic characteristics of the lucky survivors leave a big mark on further evolution.
Figure 19: Major anatomical and physiological evolutionary adaptations which are especially characteristic for Homo sapiens like an increased importance of vision rather than smell; a smaller gut; loss of body hair; evolution of sweat glands; a change in the shape of the dental arcade from being u-shaped to being parabolic; development of a chin (found in Homo sapiens alone), development of styloid processes; development of a descended larynx.
Modern humans (Homo sapiens) have been exposed several times during their existence to glacial periods characterized by the risk of a hypothermia of the brain. Such an event was the super Toba volcano eruption in Indonesia around 70,000-75,000 years ago estimated as the largest volcano eruption in the last 2 million years. It was followed on a global scale by a severe rapid glacial period of around 1,000 years which might have reduced the human world population to around 10,000 individuals. For humans and other mammalian species, a fixed brain temperature must be maintained within the internal environment despite extreme climatic variations to assure the continuity and the independence of human life. Here we show that modern humans possess a ‘chilling’ enzyme Δ12 desaturase in order to avoid the body core temperature to drop below 35.0 °C (95.0 °F). In addition, we postulate that the survivors of the Toba volcano eruption have been selected according to Darwinian thought on their ability to maintain a relatively constant brain temperature in the face of a notoriously inconstant environmental temperature characterized by extremely harsh adverse (i.e., cold) environmental conditions. Because the short severe global glacial period not only affected modern humans but also other organisms, we hypothesize that this ‘chilling enzyme’ Δ12 desaturase should not only be found in humans but also in other vertebrates and invertebrates. In this study, we also show that this ‘chilling enzyme’ is present in the brains of another mammal, the common house mouse (Mus musculus; strain C57bl6). This observation strengthens our hypothesis that the gigantic Toba volcano eruption (Figure 20) must have had an enormous impact on earthly life and that the surviving organisms (invertebrates and vertebrates) must all have come from those organisms that possessed the ‘chilling enzyme’ Δ12 desaturase, which enabled them to maintain their core temperature [128].
Figure 20: The scale of the power of the Toba Super volcano eruption in Indonesia around 70,000-75,000 years ago is visualized and compared to other historical well documented volcano eruptions like the Vesuvius volcano eruption of 79 CE and the Krakatau volcano eruption also in Indonesia in 1883 AD.
The eruption of the Toba super-volcano around 70,000- 75,000 years ago is estimated to be the largest volcanic eruption in the last 2 million years releasing an energy equivalent of about one gigaton or TNT. A comparison with other volcanic eruptions is presented in Figure 20. As a result of the enormous ash emissions [129,130], a solar eclipse occurred resulting in a rapid glacial winter lasting about 1000 years during which the temperatures on a global scale were reduced between 20 and 25 degrees Celsius, in the tropics to 7-8 degrees Celsius below zero (Figure 21).
This in turn, resulted in freezing of vegetation causing famine among animals and first humanoids and massive extinction of animal species and first humanoids.
Because of hunger and famine, the world population of early Homo sapiens may have been reduced to around 5,000- 10,000 breeding pairs and almost causing its extinction. If this applies to different species on a global scale, it is called ‘multiple genetic bottleneck theory’ (Figure 22).
It is therefore possible that humanity has survived from a population of 10,000 to the present about ≈8 billion. This thesis is supported by genetic evidence suggesting that today’s humans descended from a very small population of between 1,000 and 10,000 breeding pairs that existed about 70,000 years ago. According to the National Human Genome Research Institute (NHGRI) about 99.9 percent of the DNA sequence is identical in all people [131-133], which supports the notion of a small group of common ancestors and a ‘genetic bottleneck theory’ for Homo sapiens. So, we assume that the current world population of mankind -with its tremendous variety in ‘races’, ethnicities, and phenotypes (Figure 23)- consists of descendants of the small population that survived the outbreak of the global climate ‘glacial winter’ [134]. The Toba volcano eruption has been associated with a ‘genetic bottleneck’ in human evolution about 70,000 years ago [135,136] which was possibly the result of a serious decrease in the total human population as a result of the Toba event which is the most super volcano burst ever studied [137].
Cave and Rock paintings of early humans as reflection of their lifestyle
It has to be clear that early human symbolic behaviour like hunting in groups can be read from the archeologic record which information we will explore -because of the emergence of cave and rock art in human evolutionand assess its relation to the “psychological” supportive evidence for our “African Buffalo Savannah” hypothesis, which in this review is mainly defended based on an ecological perception but will be elucidated from a biochemical point of view in a next review manuscript (van Ginneken 2020 in press). In this respect, an extraordinary feature that people share, separating us from all other living things, is our “unique symbolic cognitive style” [138]. As the pHilosopher Ernst Cassirer pointed out, humans are not the animal rationale but the animal symbolicum [139]. Although other animals are able to challenge cognitive behavior - for example, the ability of the crow to make stick tools [140] and the apparent symbolic mediated behavior of late Neanderthal populations [141] - man’s capacity for symbolic thinking is immeasurably greater and qualitatively different, so much so that Charles Darwin himself remarked: “the difference between the mind of the lowest man and that of the highest animal is immense”.
When did we acquire this cognitive capacity for symbolic thinking? The answer to this question must necessarily be based on indirect evidence, because we have no access to facts about the variability and heredity of this trait [142]. Suppose we equate high cognitive capacity with brain size. The human-like brain has been growing for 2 million years and doubled in size twice [143] with modern people at the end of the line with the highest encephalization quotient [144]. There must be something in human evolutionary history which indicates a drastic and qualitative change in behavior i.e., the emergence of symbolic thinking. 138. Tattersall (2017) [138] makes an intriguing observation about the pace of technological innovation. The first stone tooling technology appeared 2.5 million years ago [145], and it remained essentially the same for a million years before innovation was introduced in the form of the Acheulean hand-axe. Another million years passed before an important innovation took place, in the form of core preparation. In other words, innovation was rare and alternated with long evolutionary timeperiods in which hardly any change took place. This has been acknowledged in our Human Brain Tree of Life because from Australopithecus africanus (with a brain volume of around 300-500 cm3 towards Homo habilis (with a skull volume of 600 cm3 [7] is an evolutionary timespan of around 2-2.5 million years (Figure 24) [146].
But by the end of the Pleistocene, a profound change took place: technological innovations began to appear more quickly, and this meant a “relatively abrupt and qualitative change in the processing of mental information” [147]. This can probably be explained by the evolutionary development of a six-layer neocortex (compared to the three-layered brain of a reptile) and an evolutionary evolving brain with an extremely high neural density compared to other mammals. To explain the “intelligence” property in terms of brain skull volume in evolution is too simple an approach.
In this regard, it is also important to note that the degree of brain mass or volume, seen as brain capacity, or even relative brain size - that is, brain mass expressed as a percentage of body mass - is not a measure of intelligence, nor of use or function of brain regions. However, total numbers of neurons do not correspond to a higher ranking in cognitive skills. Elephants have a higher number of neurons (257 billion) [148,149]. Relative brain size, total mass and total number of neurons are just some indicators assisting scientists in following the evolution trend of an increased brain-to-body ratio due to hominine phylogeny. In addition to size, scientists have observed changes in the folding of the brain, as well as in the thickness of the cortex. The more complex the surface of the brain, the larger the surface of the cortex, allowing an extension of the cortex, the most evolutionary advanced part of the brain [150]. A larger surface of the brain is linked to higher intelligence as is the thicker cortex, but there is a reverse relationship - the thicker the cortex, the harder it is to fold. In adult people, a thicker cortex is linked to higher intelligence. The neocortex is the most advanced and most evolutionary part of the human brain. Six layers thick and present only in mammals, it is especially prominent in humans and the location of the most functional and cognitive capacity at a higher level [151]. The six-layered neocortex found in mammals is evolutionarily derived from a three-layer cortex present in all modern reptiles [152]. This threelayer cortex is still preserved in some parts of the human brain, such as the hippocampus, and is believed to have evolved in mammals to the neocortex during the transition between the Triassic and Jurassic. The three layers of this reptile cortex are strongly related to the first, fifth and sixth layers of the mammalian neocortex [153]. Among mammals, primates have a higher neuronal density compared to rodents with similar brain mass, and this may be an explanation for increased intelligence.
Miyagawa [154] et al 2018 propose that the phenomenon of cave and rock art plausibly indicates how an internalized “system of thought” [154,155], which presumably evolved with the speciation of modern Homo sapiens around 200,000 years ago [156], may have taken shape into concrete, externalized language. If this turns out to be true, the often-stated idea that “performed action does not fossilize” is not quite true because the cave paintings are pieces of externalized “language”. Furthermore, they indicate and are a reflection of the: a) The tools/ weapons that were used; b) The complex social interaction like hunting in groups [157]; c) Were there any other (domesticated) animals used during hunting like horses; d) The prey animals that were hunted like the African buffalo (here a central theme in this review) but also other African savannah animals like the eland elephant or giraffe.
I have mainly restricted myself to cave paintings mainly showing the ancestors of the African buffalo, but the cave paintings of South Africa and the south of France also show countless animals that were hunted. Such indications from a distant past show us how the hunting process among these early hominids proceeded and largely support the predominantly biochemical evidence of the African buffalo as described further in a manuscript focusing more on lipidomics (van Ginneken 2020, in press). In addition, these cave paintings (Figure 25 & 26) might also be considered as evolutionary ‘heritage’ of the early hominids to their progeny, because these art forms express an awareness of death. By making “cave art”, early hominids created a place for themselves in the eternity of existence.
Table 2: Overview of the hominin and hominid evolutionary development of the human brain during the course of evolution over a time frame of around 5 million years.
1 |
Before 5 mya: |
In Africa, our ancestral lineage and the chimpanzee lineage split |
2 |
Before 4 mya: |
The Australopithecus anamensis walked around what is now Kenya on its hind legs. |
3 |
>3 mya: |
Australopithecus afarensis (“Lucy”) lived in Africa. |
4 |
2.5 mya: |
Some hominids made tools by chipping stones to form a cutting edge. There were perhaps four or more species of hominid living in Africa |
5 |
2 mya: |
The first members of the Homo clade, with their relatively large brains, lived in Africa. |
6 |
1.5 mya: |
Hand axes were used, Also, hominids spread out of Africa and into much of Asia and Europe. These hominids included the ancestors of Neanderthals (Homo neaderthalensis) in Europe and Homo erectus in Asia. |
7 |
100,000 |
Human brains reached the current range of sizes. Early Homo sapiens lived in Africa. At the same time, Homo neaderthalensis and Homo erectus lived in other parts |
8 |
50,000 years ago: |
Human cultures produced cave paintings and body adornment and constructed elaborate burials. Also, some groups of modern humans extended their range beyond Africa; “The great leap forwards”. |
9 |
25,000 years ago: |
Other Homo species had gone extinct, leaving only modern humans, Homo sapiens spread throughout the Old World. |
Figure 25: Aurochs were powerful animals that were much larger than normal cattle, with bulls standing almost 2 meters high on the shoulder. Perhaps the most famous image it depicts is the frieze of attacking bulls in the so-called Hall of the Bulls at Lascaux Cave in the Dordogne of France. These may have been painted around 15,000 years ago. The earliest paintings in Lascaux are scientifically dated to around 17,000 years before today.
Figure 26: Located in northern Spain, not far from the village of Antillana del Mar in Cantabria, the Upper Paleolithic cave complex at Altamira is famous for its magnificent multi-coloured cave painting, as well as its rock engravings and drawings. Paintings of a Bison (around 15,000 BCE) from the Altamira Cave Complex (northern-Spain).
Human evolutions in a nutshell
The evolution of the hominids after the split off from the chimpanzee lineage can be characterized as ‘bipedalism’ (walking on two legs), an evolutionary trait appearing prior to ‘large brains’. Based on the principle that our evolution is literally engraved into our body, I have presented a biochemical model for human brain growth, which is to my mind a new approach in the field of paleo-anthropology. In summary, the evolution of the human brain can be characterized by a very slow growth during 3.5 million years with a brain volume increase from Australopithecus africanus (with a brain volume of around 300-500 cm3 (146) towards Homo habilis (with a skull volume of 600 cm3 (7). In this ecologically tinted review -with a major emphasis on the ‘Out of Africa’ theory- I have placed major focus on the biological productivity of the African savannah in terms of ‘biomes’ and the herds of relevant prey for the first humanoids, including the African buffalo Syncerus caffer, on which mitochondrial DNA studies have been conducted by van Hooft et al 2002 [18]. Migration routes between these large herbivores of the African Savannah and the early hominids in the late Pleistocene seem to run parallel, so that one can properly speak of a hunter-prey correlation (9). It is characteristic for the human genus that dominates the areas of making and using more complex tools [158]. After the development of tools and weapons that may have played a role in hunting - resulting in a new food source of meat, brain volume increased from Homo habilis (cranium capacity of around 550 cm3) towards modern Homo erectus (cranium capacity of around 800- 1,100 cm3) over a time-frame of around 1 million years (Figure 3 & 24 & 27) followed by the growth spurt over the last 70,000 year towards Homo sapiens (cranium capacity of around 1,400-1,500 cm3) (Figure 3 & 24 & 27). Third, metacarpal styloid processes allows humans the dexterity and strength to make and use complex tools. This unique anatomical feature separates humans from apes and other non-human primates, and is not seen in human fossils older than 1.8 million years
In addition, in this review I have briefly discuss the genetic bottleneck theory for Homo sapiens from our previous own work stating that an enormous volcano eruption - the Toba volcano eruption on North Sumatra, Indonesia – presumably caused a genetic bottle neck for early Homo sapiens (128, 129).
In comparison with the genetic variation among the various other apes, the variation between humans is very small, only 0.1% (133). Scientists assume that our ancestors went through a bottleneck about 100,000 years ago after a huge volcanic eruption had drastically reduced their numbers to only a few thousand individuals around 80,000 years ago. Because all of us descend from that small group, much of the original variation has disappeared. The smaller a population, the more coincidence will play a role. An extreme example of how coincidence influences evolution is “genetic bottle necks” or ‘bottle necks’, where a large part of the population is killed and the genetic characteristics of the lucky survivors -in our lipidomics related research manuscript we hypothesize the ‘chilling’ enzyme Δ12 desaturase (128, 129) leave a big mark on further evolution.
As mentioned earlier, we have focused on the skull expansion during the course of evolution. Based on earlier archeological and anthropological data we hypothesize that the major skull expansion took place during the time period of Homo erectus, a period characterized by improved hunting and thus improved food quality including meat on a regularly daily basis –most important during infancy. Ideas of brain evolution where food quality is central have not been limited to humans and human evolution. Parker & Gibson (1978) [36] and Gibson 1986 [37] has devised the ‘Extractive Foraging Hypothesis’ to explain the relationship with primates. Gibson and coworkers argued that a relatively large brain correlates with omnivorous nutrition in primates, which requires relatively complicated strategies for extracting high-quality foods. This is probably one of the most intriguing and complex questions in the biological sciences: “What makes us human, can it be ascribed to our overgrown omnipotent brain?”
As mentioned in the introduction, presently two mainstream hypotheses related to human brain encephalization exist. On one hand there is the 50 years old Aquatic Ape Hypothesis (AAH), on the other, the terrestrial ‘Savannah’ Dry-Land based (SDL) hypothesis (60).
In the discussion about the Aquatic Phase Hypothesis vs. the Terrestrial Dry-Land Savanna Hypothesis a reasonably important question was raised by Crawford (2002) [159] viz. if Savannah food produced sufficient iodine for the developing brain of the early hominids. This is an important relevant question because Figure 28 shows indirectly - based on human data, guidelines and standards - that given the current human iodine consumption patterns of iodine fortified foods, the African continent (especially the region interior) is particularly affected by a chronic iodine deficiency [160]. If iodine is consumed in less than the recommended amounts, it may result in cognitive impairments in humans, so it not unreasonable to expect that iodine deficiency would hamper evolutionary human brain growth (encephalization). Information from hominoid primates and their diet can shed light on how prehistoric hominids improved their iodine intake. The study of [161]. Hohman et al (2019) with bonobo’s demonstrated that the lowland forest offers natural sources of iodine in concentrations high enough to prevent iodine deficiency in hominoids and humans. Especially two herbs stand out for their high iodine concentration: the terrestrial herb Haumania liebrechtsiana with concentrations in the range of 0,3-0,36 mg/kg dry weight and Drypete spp with concentrations of around 0,1 mg/kg dry weight.
An interesting observation for the great apes of the savanna is that Bonobos eat less meat than chimpanzees yet acquire their nutritional required protein amount (but also iodine) by eating the savannah herb Haumania liebrechtsiana. This herb is abundant in their habitat in contrast to the habitat of the Chimpanzees, which is located in the stable forests of central and western Africa which provide a stable environment containing food in abundance. Presently, human proximity and habitat fragmentation are key drivers of the range-wide bonobo distribution.
Genomics versus lipidomics
In a ground-breaking 1975 paper published in Science, evolutionary biologist Allan Wilson of the University of California (UC), Berkeley, and his graduate student Mary-Claire King argued convincingly for a 1% genetic difference between humans and chimpanzees [163]. The International Scientific Society was shocked because the genetic distance was so small for such clearly different species. In fact, this 1% represents the frequency of point mutations which occurred after the divergence of the human and chimpanzee lineages, but other genetic events, such as insertions and deletions of small DNA fragments and gene duplication, must also be taken into account to faithfully describe the genetic difference between humans and chimpanzees [164].
Since the Seventies of previous century, it has been claimed that genome studies can in principle provide exciting hints about what makes a human brain unique. The new studies rely on genome sequence data from the closest family members of humans in comparison with other primates. The data allow scientists to compare DNA from e.g. chimpanzees and humans and to look for signals (specific sequences or sequence elements) that seem unique to humans. Human-specific social and cognitive behavior, including language, civilization, society, as well as some mental disorders, are rooted in the complex human brain. The mechanisms underlying human-specific neurological development, however, remain unclear. Clarifying the relationship between genetic mutations and human-specific characteristics and function, compared to non-human primates, is a primary goal of brain evolution studies. Previous studies have provided strong evidence that brain-related genes play an important role in the evolution of brain differences between humans and other primates. Several factors, including epigenetic and posttranscriptional modification, and differences in expression profiles during different stages of brain development contribute to the differences observed among primate brains. Unlike variations in coding regions, which usually lead to loss or gain of gene product function, mutations in non-coding regions can affect the binding affinity of transcription factors or the recruitment of transcriptional elements, thereby influencing the expression of downstream genes. Non-coding regions are involved in various regulatory processes, including cleaving premRNAs during transcription, assisting mRNA localization, and transcription.
Xu et al. [165] analyzed the transcription of intergenic and repeated regions in human, chimpanzee and macaque brains and discovered that intergenic transcripts exhibited more expression differences between species than exons, demonstrating the importance of regulatory elements in brain-related differences between species. Different human genomic regions displaying accelerated evolution are associated with neurological development, cognition, social behavior and even brain disorders [166, 168, 169]. These findings provide powerful evidence of the importance of non-coding regions in the development and divergence of primates, as well as human brain diseases. A more extensive analysis should be conducted to determine how regulatory regions in the genome participate in the evolution of the primate brain and which biological functions they serve. DNase I hypersensitive sites (DHSs), i.e., sites in which the chromatin structure has been altered thus that the DNA is exposed and accessible, contain a variety of regulatory elements, including promoters, enhancers, silencers and other transcription factor (TF) binding sites. A wider question raised by this genomics work and other recent studies is how one can find out what exactly the newly discovered genes do [170]. With around 30,000 genes in the human genome (National Human Genome Research Institute 2019), this seems to be a daunting task because a gene under survey can only be expressed in a rodent model of hopefully to study its possible role in brain encephalization. However, with the development of the ENCODE project and high-throughput DHS detection methods, hundreds of DHS data sets are available online, providing access to high-resolution network information. In addition, recent studies on the evolution of DHS have shown that these regions play an important role in the alteration of gene regulation and therefore affect human-specific traits and their development. Analysis of the accelerated evolution of these regulatory elements that control brain development genes, reveals the genetic mechanisms that underlie functional differences between the brains of humans and non-human primates. In the study of Lu et al (2018) (166) DHSs were identified in the regulatory regions of genes related to brain development, especially of those brain regions that show most divergence between primates, such as the cortex. DHSs exhibiting accelerated evolution (aceDHSs) were identified in brain-related gene-regulatory regions. Changes in these regulatory regions, notably SNPs, play an important role in establishing differential expression patterns among primates and the occurrence of brain disorders (166).
Ultimately, however, understanding the evolution of the human brain will depend on more than sequence data [171]. Genetic modifications identified by sequence comparison must be transcribed in phenotypic terms. In the case of the brain, this requires detailed functional analysis spanning molecular, cellular and system neurobiology. Simple explanations of human uniqueness based on general notions of increased brain size and complexity are no longer sufficient.
Maybe we should start this attempt in unraveling ‘the mysteries of mysteries’ with the simple observation that the human brain consists of nearly 60 percent fat (66). Hence, a lipidomics approach could be the most favorable approach in tackling this evolutionary problem (to say it in simple terms). lipidomics is the large-scale study of pathways and networks of cellular lipids in biological systems. The word “lipidoma” is used to describe the complete lipid profile in a cell, tissue, organism or ecosystem and is a subset of the “metabolome” that also includes the other three main classes of biological molecules: proteins / amino acids, sugars and nucleic acids. lipidomics is a relatively recent research field that has been driven by rapid advances in technologies such as mass spectrometry (MS), nuclear magnetic resonance (NMR) spectroscopy, fluorescence spectroscopy, double polarization interferometry and computational methods, and linked to the recognition of the role of lipids in many metabolic diseases such as obesity, cardiovascular diseases and type 2 diabetes. This fast-growing field complements the enormous progress that has been made in the field of genomics and proteomics, all of which form the family of Systems Biology [172]. To see it all in a historical context: the end of the 20th century was marked by the genomics revolution, and one might say that the beginning of the 21st century is marked by efforts to bring our knowledge of a cell’s proteins, known as proteomics, on a par with our growing knowledge of a cell’s transcripts, known as transcriptomics. Many of us believe that the next evolution of the omics revolution will be to map all metabolites of a cell, known as metabolomics. Leading the way in these efforts is the work in many laboratories on one subset of the metabolome, the lipidome, aimed at mapping all lipids of a cell, known as lipidomics [173]. The ultimate goal is to evolve into an integrated omics picture (the “interactome”) of genes, transcripts, proteins, and metabolites fully describing cellular functioning [174]. One leading effort in the lipidomics evolution on the human brain was performed earlier in our laboratories. We will provide a summary of the results obtained during our research of the past three years and based on it give an evolutionary plausible explanation for the overgrown neocortex of the human brain, “the mysteries of mysteries.” We think we are able to do so, because landmark discoveries in biology and (bio) medicine are more often a matter of serendipity than of conscious decision. Our explanation is based on observations never before described of an obese C57bl6 mouse model with an overgrown brain (“brain steatosis”) due to accumulation of Triacylglycerols (TGs) from bovine lard in the brain from the feed (High-fat diet) during their juvenile growth phase. To our knowledge brain expansion due to nutritional intervention has never before been observed in a mouse model related to TGs accumulation due to a High-fat diet based on bovine lard. In a followup review, we will expand this observation towards the research area of paleo-anthropology and “encephalization” of early hominids (van Ginneken 2020 in press). We are aware that we are no specialists in the research field and that human evolution is to some extent totally new to us. However, our lipidomics based observations could be valuable for the International Scientific Community (ISC) because just like modern mitochondrial DNA techniques gave a major “Leap Forward” in the research area of paleo-anthropology, this could also be the case with using a Systems Biology like lipidomics research boosting our understanding of chronic degenerative welfare diseases often related to a High-fat diet like obesity [97, 175] hepatic steatosis (≈liver disease), cardiovascular diseases (CVDs) cancer, human brain pathology (Type-2 diabetes & Type- 3 diabetes) (mental disorders and evolutionary [176-184] based approach using LC-MS techniques in a C57bl6 mouse model or post mortem human brains. In our view, evolution is literally engraved in our human brain (182). Via a lipidomics based approach we hope to provide supportive evidence for the evolutionary urgent compelling question to explain the “mysteries of mysteries”, the overgrown human brain, and to contribute to the formulation of the ultimate evolutionary model explaining the excessive brain growth (encephalization) (Figure 29); “Where Darwin neglected to explain the human brain encephalization”.
Conclusions & Perspectives
The evolution of the hominids after the split from the chimpanzee lineage can be characterized by ‘bipedalism’ (walking on two legs; Figure 12 & 27) an evolutionary trait appearing prior to ‘large brains’. Based on the principle that our evolution is literally engraved into our body, I have earlier in this review given sound ecological arguments for the tremendous productivity of the African savannah with its tremendous herds of the early African buffalo’s (Syncerus caffer) for human brain growth. Through this ecological approach, I hope to contribute to a further deepening in the research field of Paleo-anthropology.
In summary, the evolution of the human brain can be characterized by a by a very slow hominin brain growth during 3.5 million years with a brain volume increasing from that of Australopithecus africanus (with a brain volume of around 300-500 cm3 [146] towards that of Homo habilis (with a skull volume of 600 cm3 [7]. Homo sapiens have lived from about 250,000 years ago to the present. Between 400,000 years ago and the second interglacial period in the Middle Pleistocene, around 250,000 years ago, skull expansion and the elaboration of stone tool technologies, provided evidence characterized by a transition from Homo erectus to Homo sapiens (Figure 3 & 27).
Former assumptions suggested there was a migration of Homo erectus out of Africa, followed by a further speciation of Homo sapiens from Homo erectus in Africa. A subsequent migration within and ‘Out of Africa’ model of monogenesis for the pattern of human evolution eventually replaced the earlier dispersed Homo erectus model. This migration and origin theory is usually referred to as the “Recent Single Origin” or “Out of Africa” theory [9,17,27,28].
During the last 20 years, the theory of a recent African origin has become the leading idea for developing a new overarching model for the evolution of modern humans. This theory argues that we recently originated in Africa, moved out of this continent, and replaced all of the other human forms outside of Africa. Current excavations of fossils containing sequencable amounts of DNA offers however an exciting new perspective to the ‘evolution sciences’ of modern humans. Presently, the theory of ‘Multiregional Evolution’ [187-192] is on the rise and supported by molecular genetic research it offers a modern version of the ‘Out of Asia’ [193]. Yan Dejian et al 2018 [193] concept because of genetic data suggest that modern archaic man coming out of Africa about 60,000 years ago probably interbred first with Neanderthals, and then later some of them interbred with another group of humans called the Denisovans, somewhere in south-eastern Asia.
The multiregional hypothesis was first proposed in 1984 ([189]. Wolpoff et al. 1984), and then revised in 2003 (192). In its revised form, it is similar to the “assimilation model,” which states that modern man come from Africa but also underwent small, geograpHically variable, degrees of mixing to of other regional (archaic) hominids [194]. Presently, there are four basic models claiming to explain the evolution of Homo sapiens between approximately 315 and 30 kya. At the one extreme of the spectrum, there is ‘multiregional evolution’, or the ‘regional continuity model’ [188-190]. At the other extreme, there is the African replacement model’’, or ‘Out of Africa model’ [26, 192]. In between these extremes, we have the African model for hybridization and replacement and the assimilation model [187]. Anything, but the multiregional model claims that Homo sapiens evolved exclusively in Africa and was subsequently deployed to Eurasia and finally to America and Oceania. Both replacement models claim that anatomically modern emigrants have replaced resident Eurasian and Australasian species Homo sapiens with little or no hybridization. The hybridization and replacement model suggest some crossbreeding with archaic indigenous populations but with relatively small effects. Assimilation maintains continuity between archaic and modern people, particularly in some areas of Eurasia, where gene flow and local selective factors would also cause morphological changes. In this model, the unity of the species was maintained by periodic crossing over large areas. Multi-regionalists reject the idea that Homo sapiens has uniquely evolved in Africa. Instead, they argue that discrete archaic populations of Homo developed locally in Africa, Asia, and Europe. During their tenures, both archaic and descendants were interbred with contemporaries from other areas[194]. Here, we present a fifth model, taking into account the effects of a global mass extinction among early hominids due to the Toba volcano eruption [128-132].
However, current genetic DNA evidence does not preclude some multiregional evolution or some admixture of the migrant Homo sapiens with existing Homo populations [187]. Briefly, ‘Multiregional evolution’ is a model to explain the pattern of human evolution in the Pleistocene. The underlying hypothesis is that one global network of genetic exchanges between evolving human populations that constantly share and reticulate, provides a framework of population connections that makes both species-wide evolutionary change and locally possible distinction and differentiation possible. “Multiregional” does not mean independent multiple origins, old divergence of modern populations, simultaneous appearance of adaptive characters in different regions, nor parallel evolution [190].
Yet the ‘Out of Africa’ theory still is the most credibile of the human evolution theories because it is supported by: a). Observations on huge amounts of fossils of cranium (skulls), bones and fossil features identifying them as possible tools or weapons; b). Ecological support for the production of a large quantity of meat on the large African savannah - a continuous highly productive terrestrial ecosystem of hundreds of square kilometers - also referred to as ‘biomes’; c) [14]. The ‘African Buffalo Savannah hypothesis’ (‘ABS hypothesis’), described in this review which provides strong evidence that the meat and bovine lard of the herds of the African buffalo on the African savannah in the Pleistocene had such a specific biochemical composition that they could have been the evolutionary driving force for encephalization of the early hominids (van Ginneken 2020, in press); d). The previously published map of migration routes of the early African buffalo (ancestors of Syncerus caffer) and early hominids - hunter versus prey correlation - which provides us with supporting evidence for the ‘ABS hypothesis’; [9] e). Cave-paintings of around 20,000-30,000 years old, reflecting ancestors of the African buffalo (Syncerus caffer). Other regions or even continents such as Europe, Siberia, China and Southeast Asia lack such direct indications of a certain mental ability for symbolic art of these early hominids. In addition, these cave paintings provide insight into various aspects of the ‘hunter-gatherer society’ at that time. Yet, lack of these cave paintings doesn’t support a ‘Multiregional evolution’ theory.
While writing this review, the following personal view on the evolution of the Homo sapiens sapiens emerged which I will briefly summarize here. In this review, I presented a broad overview of the evolution of the first hominids with a strong emphasis on the ecological ‘carrying capacity’ of ‘biomes’ around the world to produce sufficient prey animals (‘≈ meat’) for the developing brain of early hominids (Homo habilis and Homo erectus), thus considering ‘meat’ as a inherent evolutionary driving force for human brain growth The ecology (‘biomes’) of the African savannah has produced enough meat (in particular from the African buffalo Syncerus caffer) to increase the brain volume of Homo erectus (800-1,100 cm3) in about 75,000 years nearly a doubling to the modern brain of ‘1,500 cm3’ of Homo sapiens sapiens. Nearly 20 years ago ([26]. Stringer 2003) presented, based on fossil record findings in Ethiopia, very strong evidence for the ‘Out of Africa theory’. This was followed by genomic DNA studies supporting this view [192]. Nearly twenty years later we state here, based predominantly on strong ecological arguments (to be followed up by a biochemical review van Ginneken 2020 in press), that the cradle of Homo sapiens sapiens can only have been/stood at the African continent with its tremendous carrying capacity for prey animals in the ‘biomes’ of the African savannah. This is not solely a view of major scientific importance but has also carrying a strong political message for the whole African continent! (the sleeping lion arises).
Subsequently, various waves of expansion of hominids took place from Africa to other continents [9] probably accompanied by a mixing with local hominids (multiregional hypothesis) [187-190]. However, this mixing of several early Homo species may be indicated by regional fossils, but not in the genetic material of modern hominids.
Especially amongst ‘genomics’ researchers, recent human evolution is an area of great controversy [195]. As an example, the publication of a haplotype tree based on human mitochondrial DNA variation in 1987 (196]. Cann et al 1987) elicited a fierce debate about the details of recent human evolution that continues to this day, as did studies of African populations based on variation in mitochondrial DNA [197]. Despite these continuing controversies, the International Scientific Community has reached general consensus on human descent in Africa and the spread to South Eurasia as Homo erectus. After Homo erectus had spread from Africa, the replacement model outside of Africa [198] asserts that populations in Africa, Europe and Asia had little genetic contact and evolved independently, with anatomically modern humans evolving only in Africa. This theory is in line with our ‘African Buffalo Savannah’ hypothesis model, as described in this review manuscript, which argues that human evolution towards modern archaic Homo sapiens sapiens with its modern ‘1500 cm3’ brain took place at the African savannah.
After the evolution of modern humans in Africa, a second expansion took place in from Africa about 100,000 years ago, resulting in the worldwide replacement and genetic extinction of non-modern human populations by anatomically modern humans [199]. Under the multiregional evolution model [190] genetic contact between African and non-African Homo erectus populations was maintained, although limited by remote isolation. Remote isolation allowed local populations to be distinguished from one another, but gene flow prevented long-term independent evolution such that humanity evolved into modernity along a single evolutionary line [192].
It is possible that the current world population of 7.8 billion people is the offspring of only about 1,000 to 10,000 breeding pairs that survived after the short ice age which lasted about 1,000 years after the huge Toba volcano eruption took place about 80,000 years ago [128,129,135,136], This theory is supported by the fact that the differences in the DNA of current humanity amount to only 0.1% percent [133]. How can we explain so many different species, varieties, ethnicities among Homo sapiens in light of these findings? I propose to compare these findings with the aforementioned important observation from 1975 by [163], who found only 1% difference in genetic material between the chimpanzee and modern humans. The enormous variety of people types, ‘races’, and ethnicities can only be explained by considering the genetic mechanisms causing this 1% difference at the primary DNA level, such as insertions and deletions of small DNA fragments and gene duplication as well as their consequences for gene expression, in order to faithfully describe the genetic difference between humans and chimpanzees [164]. In this way one should also consider the tremendous amount of varieties of human species (Figure 23) of ‘races’, species, ethnicities, of modern humans which solely differ for 0.1% in their DNA (whole genome sequencing Human Genome Project; 133).
So, the multi-regional theory, based on the fossil record before 80,000 years ago characterized by the Toba volcano eruption event and its subsequent genetic bottleneck theory - which decimated humanity towards 1,000 – 10,000 breeding pairs- cannot be sustained. So, I postulate that all excavations of the fossil record of hominids before 80,000 years ago are “really dead fossils” because their DNA has not been passed on to future generations. According to this genetic bottle-neck theory solely the 1,000-10,000 breeding pairs -survivors of the Toba volcano eruption- were able to pass on their DNA to the next generation. If the 1,000-10,000 breeding pairs (Toba volcano survivors) were not solely restricted to the south-east Asian region but were scattered all over the world (Europe, Siberia, China, Southeast Asia and, last but not least, Africa), then regional differences in the DNA of these hominids can be based on mixing earlier with other hominids (Neanderthals and Denisovans) explaining the tremendously increasing genetic variation. Only within the boundaries of this scenario, the ‘Multiple Region theory’ can hold true with the 0.1% variation among humans at the DNA level explaining the diversity of ‘races’, species, ethnicities of modern man.
The awareness ‘that human evolution is literally engraved into our bodies’ is an important new approach in human evolutionary sciences which we will join in order to describe the encephalization process of the human brain during the course of evolution. From this starting point, we will describe in a next review (van Ginneken 2020, in press) our findings about the biochemical composition of the human brain following a Systems Biology (172]. Kitano 2002) lipidomics based approach.
What is known about the brain is a scanty amount of information despite its importance in human evolution. As described, cranial capacity increased over a period of 700,000 years from 850 cm3 towards ≈1,500 cm3 (Figure 3) ([101]. Stringer 1994) which is equivalent to every generation having an additional 125,000 neurons more than their parents, but what were the building blocks for these neurons? It is a daunting task to describe the human brain based on biochemical composition of each cell type. In the next review manuscript (van Ginneken 2020, in press) we will describe how we attempt, by means of a holistic Systems Biology lipidomics approach with LCMS techniques, to determine the lipid composition of the brain using the homogenate of a part (gyrus) of the neocortex of post mortem material of the “Dutch Brain Bank” [182]. This investigation will be supported by similar LCMS measurements in a C57bl6 mouse model [16]. From an evolutionary point of view, the occurrence of the “fish oils” in blood and/or brain is interesting as is their ability to pass the Blood Brain Barrier (BBB) (van Ginneken 2020, in press). Based on our observations, some of these Very Long Chain Fatty Acids (VLCFA) might have played a role in human brain evolution, similar to that found for our C57bl6 mouse model which developed ‘brain steatosis’ due to a High Fat diet on 24% bovine lard figure 9; 10 [9]. van Ginneken et al 2017). In addition, we will also address the question which lipid class-the Poly Unsaturated Fatty Acids (PUFAs) vs. the Triacylglycerols (TGs) - might have played a major role in the process of human brain growth and encephalization [200-202] (Figure 30).
Finally, as Science has progressed towards an Systems Biology approach of evolutionary science (Figure 25) and as we further move towards an understanding of ourselves, the way we sit, see, walk, sleep, hear, breath and eat (the wonderful adaptation of living things), how all these things could have developed without any plan, without any benevolent Supreme Being, without a guiding hand, but through a conclusively endless series of ‘breedings’ and ‘eatings’ out of which some traits were developed and became what we see today. This is a little debunking, that human beings are not ‘the stars of a cosmic drama’ that has been planned from the beginning but are part of a world governed by impersonal forces. A world in which ‘by accident’ plays a large role, a world in which the fundamentals of ‘the mysteries of mysteries’, the human omnipotent brain, is just based on these selected traits during the long course of human evolution (Citation: Prof. Dr. Steven Weinberg, Dutch Television, VPRO-series, Beauty and Consolation, Wim Kayzer).
Therefore, a ‘Systems Biology Approach’ (172]. Kitano 2002) on Human Evolution is clearly warranted facing the blending of several research fields giving tremendous datasets related to ecological-, anatomical-, physiological-, biochemical-, (bio)medical and nutritional data (‘the modern synthesis of understanding’ Huxley 1187-1975 Finally, the mechanism of epimutations in the germline that become permanently programmed can allow transmission of transgenerational phenotypes a process called epigenesis. Epigenesis is defined as heritable changes in gene expression that does not alter DNA sequence but are mitotically and transgenerational inheritable [202] (Figure 31).
Acknowledgments
Dr. Hans Lammers is kindly acknowledged for helping to write the genetic DNA session in this review manuscript under §10 and peer reviewing of this manuscript while Floris Schouten is also kindly acknowledged for drawing and providing figure 12 & figure 31. Furthermore, I’m very grateful to Jaap & Marita van Meerveld for continuous help and support.
References
- Lamarck JB de (1809). pHilosopHie zoologique, ou Exposition des considérations relative à l'histoire naturelle des animaux, Dentu.
- Darwin C (1859). ”On the Origin of Species”; [edited by Quammen D (2011), Sterling Publishing, New York]; ISBN 978-1-4027-8959-5; 544 pages.
- van Ginneken V (2019). [Case Report]: Hunger-Obesity Paradox: The 21st Century and the “Struggle for Survival” for Whole Populations. Gastroenterology & Hepatology International Journal 4(2): 1-4; ISSN: 2574-8009; DOI: 10.23880/ghij-16000155.
- Darwin C (1871). The Descent of Man, and Selection in Relation to Sex; Publisher: John Murray; Publication date: 24 February 1871; Media Type: Print (hardback); two 450-page volumes.
- Mafart B, Guipert G, De Lumley M-A, Subsol G (2004). Three-dimensional computer imaging of hominid fossils: a new step in human evolution studies. Can Assoc Radiol J 55(4): 264-270.
- Eccles JC (1989). Evolution of the Brain: Creation of the Self; Routledge, XV + 282 pp.
- Herculano-Houzel S (2012). ‘Hominin Evolution: Estimates of Numbers of Brain Neurons in Prehistoric Homo’; Chapter 1: 2-12; Book: “Brain Evolution”; The Human Nervous System, third edition; Eds. JK Mai & G Paxinox, 2012; Elsevier inc.
- Hofman MA (2014). Evolution of the human brain: when bigger is better. Frontiers in Neuroanatomy 8: 15 pp; ISSN: 1662-5129; doi: 10.3389/fnana.2014.00015.
- van Ginneken V, van Meerveld A, Wijgerde T, Verheij E, de Vries E, van der Greef J (2017). [Hypotheses]: Hunter-prey correlation between migration routes of African buffaloes and early hominids: Evidence for the “Out of Africa” hypothesis. J Mol Med 4(3):1-5.
- Dart R (1925). Australopithecus africanus: The Man-Ape of South Africa. Nature 115 (2884): 195–199.
- Alemseged G & Zeresenay D (2017). Australopithecus afarensis Scapular Ontogeny, Function, and the Role of Climbing in Human Evolution. Science 338 (6106): 514–517.
- Cerling TE, Wynn JG, Andanje SA, Bird MI, Korir DK, Levin NE, Mace W, Macharia AN, Quade J, Remien CH (2011). Woody cover and hominin environments in the past 6 million years. Nature 476(7358): 51-56; doi:10.1038/nature10306.
- Domínguez-Rodrigo M (2014). Is the “Savanna Hypothesis” a Dead Concept for Explaining the Emergence of the Earliest Hominins? Current Anthropology 55(1): 59-81.
- Paine, O. C. C., Koppa, A., Henry, A. G., Leichliter, J. N., Codron, D., Codron, J., … Sponheimer, M. (2019). Seasonal and habitat effects on the nutritional properties of savanna vegetation: Potential implications for early hominin dietary ecology. Journal of Human Evolution, 133: 99–107.
- Klein RG & Cruz-Uribe K (1991). The bovids from Elandsfontein South Africa and their implications for the age paleo-environment and origins of the site. The African Archaeological Review 9: 21-79.
- van Ginneken V, de Vries E, Verheij E, van der Greef J (2017). ‘Brain steatosis’ in an obese mouse model during cycles of Famine and Feast: the underestimated role of fat (WAT) in brain volume formation. Integr Mol Med 4(2): 1-6. ).
- Smitz N, Berthouly C, Cornélis D, Heller R, Van Hooft P, et al. (2013). Pan-African Genetic Structure in the African Buffalo (Syncerus caffer): Investigating Intraspecific Divergence. PLOS One 8: e56235.
- van Hooft WF, Groen F, Prins HHT (2002). Phylo-geography of the African buffalo based on mitochondrial and Y-chromosomal loci: Pleistocene origin and population expansion of the Cape buffalo subspecies. Mol Ecol 11: 267-279.
- Dunbar, R. I. M., & Shultz, S. (2017). Why are there so many explanations for primate brain evolution? pHilosopHical Transactions of the Royal Society B: Biological Sciences, 372(1727), 20160244. doi:10.1098/rstb.2016.0244
- Megaze, A., Balakrishnan, M., & Belay, G. (2017). Current population estimate and distribution of the African buffalo in Chebera Churchura National Park, Ethiopia. African Journal of Ecology, 56(1), 12–19. doi:10.1111/aje.12411
- White TD, Suwa G, Asfaw B (1994). Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature 371(6495): 306–312.
- White TD, Asfaw B, Beyene Y, Haile-Selassie Y, Lovejoy CO, Suwa G, WoldeGabriel G (2009). Ardipithecus ramidus and the Paleobiology of Early Hominids. Science 326(5949): 75-86.
- Alemseged G & Zeresenay D (2017). Australopithecus afarensis Scapular Ontogeny, Function, and the Role of Climbing in Human Evolution. Science 338 (6106): 514–517.
- Haile-Selassie Y, Melilla SM, Vazzana A, Benazzi S, Ryan TM (2019). [Article]: A 3.8-million-year-old hominin cranium from Woranso-Mille, Ethiopia. Nature.
- Gonder MK, Mortensen HM, Reed FA, de Sousa A, Tishkoff SA (2007). Whole-mtDNA genome sequence analysis of ancient African lineages. Mol Biol Evol 24: 757–768.
- Stringer C (2003). Human evolution: Out of Ethiopia. Nature 423: 692-693, 695.
- Mellars P (2006). A new radiocarbon revolution and the dispersal of modern humans in Eurasia. Nature 439: 931-935.
- Meredith M (2011). Born in Africa: The Quest for the Origins of Human Life New York: Public Affairs ISBN 1-58648-663-2.
- Wood B & Collard M (1999). The human genus. Science 284: 65-71.
- Rightmire GP (2013). Homo erectus and Middle Pleistocene hominins: brain size, skull form, and species recognition. J Hum Evol 65(3): 223-253.
- Cosgrove KP, Mazure CM, Staley JK (2007). Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiat. 62 (8): 847–855.
- van Ginneken V, Verheij E, Poelmann R, Ramakers R, van Dijk KW, Ham L Voshol P, Havekes L, van Eck M, van der Greef J (2007). Metabolomics (liver and blood profiling) in a mouse model in response to fasting: a study at hepatic steatosis. Biochimica Biophysica Acta 1771: 1263-1270.
- Williams MF (2002). Primate encephalization and intelligence. Hypotheses 58: 284–290.
- Aiello LC (1997). Brains and guts in human evolution: The Expensive Tissue Hypothesis. Current Anthropol 36: 199-221.
- van Ginneken V.J.T. (2019). A stochiometric model for Human Brain Evolution. EJPMR 6: 221-230.
- Parker ST & Gibson KR (1979). A developmental model for the evolution of language and intelligence in early hominids. Behav Brain Sci 2: 367-407.
- Gibson KR (1986). Cognition brain size and the extraction of embedded food resources, In: Primate Ontogeny Cognition and Social Behaviour (Else JG & Lee PC eds) Cambridge University Press Cambridge pp 93-105.
- Leonard WR & Robertson ML (1992). Nutritional requirements and human evolution: A bioenergetics model. Am J Hum Biol 4: 179-195.
- Ardrey R (1961). African Genesis Dell New York.
- Washburn SL & Lancaster CS (1968). The evolution of hunting, In: Man the Hunter (Lee RB & DeVore I eds) Aldine New York pp 293-303.
- Isaac GL (1971). The diet of early man: aspects of archaeological evidence from lower and middle Pleistocene sites in Africa. World Archaeol 21: 278-299.
- Tanner NM (1981). On Becoming Human. Cambridge University Press, Cambridge.
- Foley RA & Lee PC (1991). Ecology and energetics of encephalization in hominid evolution. pHilos Trans R Soc Lond B 334: 223-232.
- Leonard WR & Robertson ML (1994). Evolutionary perspectives on human nutrition: The influence of brain and body size on diet and metabolism. Am J Hum Biol 6: 77-88.
- Young TP, Palmer TM, Gadd ME (2005). Competition and compensation among cattle, zebras and elephants in a semi-arid savannah in Laikipai, Kenya. Biological Conservation 122: 351-359.
- Forseth I (2010).Terrestrial Biomes. Nature Education Knowledge 3(10):11.
- Alister H (1960). Was Man More Aquatic in the Past? New Scientist 7 (174): 642–645.
- Hardy A (1960). Was Man more Aquatic in the Past? New Scientist 7(174): 642-645.
- Foley R, & Lahr MM (2014). The role of “the aquatic” in human evolution: Constraining the aquatic ape hypothesis. Evolutionary Anthropology: Issues, News, and Reviews, 23(2): 56–59.
- Broadhurst CL, Wang Y, Crawford MA, Cunnane SC, Parkington JE, Schmidt WF (2002). Brain-specific lipids from marine, lacustrine, or terrestrial food resources: potential impact on early African Homo sapiens. Comparative Biochemistry and Physiology Molecular Biology Part B 131: 653-673.
- Bradbury J (2011). Docosahexaenoic acid (DHA): an ancient nutrient for the modern human brain. Nutrients 3(5): 529–554.
- Brenna JT & Carlson SE (2014). Docosahexaenoic acid and human brain development: evidence that a dietary supply is needed for optimal development. J Hum Evol 77: 99-106.
- van Ginneken VJ, Helsper JP, de Visser W, van Keulen H, & Brandenburg WA (2011a). Polyunsaturated fatty acids in various macroalgal species from North Atlantic and tropical seas. Lipids in health and disease, 10 (104): 8pp.
- van Ginneken V, Verheij E, van der Greef J (2019). “Foetal Origins of Mental Diseases” Hypothesis Explained by a MUFAs towards PUFAs Obstruction via the Enzyme Stearoyl CoA Desaturase (SCD) in Post-Mortem Human Brain. EC Physiology and Psychiatry6 (2019).
- Uauy R & Dangour AD (2006). Nutrition in brain development and aging: a role of essential fatty acids. Nutrition Reviews 64(5): S24-S91.
- Tau GZ & Peterson BS (2010). Normal Development of Brain Circuits. Neuropsychopharmacology 35 (1): 147–168.
- Blakemore SJ (2012). Imaging brain development: the adolescent brain. Neuroimage 61 (2): 397–406.
- Johnson SB, Blum RW, Giedd JN (2009). Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Health 45 (3): 216–221.
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, Sharma S (2013). Maturation of the adolescent brain. Neuropsychiatr Dis Treat 9: 449–461.
- Tuomisto H, Tuomisto M & Tuomisto JT (2018). How scientists perceive the evolutionary origin of human traits: Results of a survey study. Ecology and Evolution 8: 3518–3533.
- McHenry HM (2009). Human Evolution. In Michael Ruse & Joseph Travis. Evolution: the first four billion years. Cambridge, Massachusetts: The Belknap Press of Harvard University Press. p. 265. ISBN 978-0-674-03175-3.
- Striedter GF (2005). Principles of brain evolution. Sinauer Associates, Inc., Sunderland, MA.
- Tocheri MW, Orr CM, Jacofsky MC, & Marzke MW (2008). The evolutionary history of the hominin hand since the last common ancestor of Pan and Homo. Journal of anatomy 212(4): 544-562.
- Ward CV, Tocheri MW, Plavcan JM, Brown FH., & Manthi FK (2014). Early Pleistocene third metacarpal from Kenya and the evolution of modern human-like hand morphology. Proceedings of the National Academy of Sciences of the United States of America, 111(1): 121–124.
- Schoenemann PT (2006). Evolution of the Size and Functional Areas of the Human Brain. Annu. Rev. Anthropol 35: 379–406.
- Chang CY, Ke DS, Chen JY (2009). Essential fatty acids and human brain. Acta Neurol Taiwan 18(4):231-241.
- Dings J, Meixensberger J, Jäger A, et al. (1998). Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery 43:1082–1095.
- Satriotomo I, Nichols NL, Dale EA, Emery AT, Dahlberg JM, Mitchell GS (2016). Repetitive Acute Intermittent Hypoxia increases growth/neurotropHic expression in non-respiratory motor neurons. Neuroscience 3322: 479-488. doi.10.1016/j.neuroscience.2016.02.060.
- Ropper AH & Brown RH (2005). Adams and Victor's Principles of Neurology McGraw-Hill Professional; 8 edition, Chapter 30 page 530.
- Wright BL, Lai JT, Sinclair AJ (2012). Cerebrospinal fluid and lumbar puncture: a practical review. Journal of Neurology 259 (8): 1530–1545.
- Hosoido T, Mori F, Kiyoto K, Takagi T, Sano Y, Goto M, Nakajima K & Wada N (2013). Qualitative Comparison between Rats and Humans in Quadrupedal and Bipedal Locomotion. Journal of Behavioral and Brain Science 3: 137-149.
- Malm J, Kristensen B, Fagerlund M, Koskinen LO & Ekstedt J (1995). Cerebrospinal fluid shunt dynamics in patients with idiopathic adult hydrocephalus syndrome. Journal of Neurology, Neurosurgery, and Psychiatry 58(6): 715–723.
- Price TD & Feinman GM (2003). Images of the Past, 5th edition. Boston: McGraw Hill. p. 68.
- CSF (1979). The significance of the evolution of the cerebrospinal fluid system. Annals of the Royal College of Surgeons of England 61(5): 349-356.
- Willie CK & Smith KJ (2011). Fuelling the exercising brain: a regulatory quagmire for lactate metabolism. The Journal of physiology 589(Pt 4): 779-80.
- Clark DD & Sokoloff L (1999). In: Basic Neurochemistry: Molecular, Cellular and Medical Aspects, eds. Siegel, G. J., Agranoff, B. W., Albers, R. W., Fisher, S. K. & Uhler, M. D. (Lippincott, pHiladelpHia), pp. 637–670.
- Salway JG (2006). Medical Biochemistry at a glance; Blackwell Publishing LTD, ISBN-13:978-4051-1322-9, 144 pp.
- Li M, Tang Y, Yao J (2018). Photo-acoustic tomography of blood oxygenation: a minireview. Photo-acoustics 10: 65-73.
- Proia P, Di Liegro CM, Schiera G, Fricano A & Di Liegro I (2016). Lactate as a Metabolite and a Regulator in the Central Nervous System. International Journal of Molecular Sciences 17(9): 1450.
- Harris T, Azar A, Sapir G, Gamliel A, Nardi-Schreiber A, Sosna J, Gomori JM, Katz-Brull R (2018). Real-time ex-vivo measurement of brain metabolism using hyperpolarized [1-13C] pyruvate. Nature Scientific Reports 8:9564.
- Smith D, Pernet A, Hallett WA, Bingham E, Marsden PK & Amiel SA (2003). Lactate: A Preferred Fuel for Human Brain Metabolism in vivo. Journal of Cerebral Blood Flow & Metabolism 23(6): 658–664.
- Spence AM, Graham MM, Muzi M et al. (1997). Feasibility of imaging pentose cycle glucose metabolism in gliomas with PET: studies in rat brain tumour models. J Nucl Med 38:617– 624.
- BLM (2011). “Brain lactate metabolism: the discoveries and the controversies” Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 32(7): 1107-1138.
- Pellerin L & Magistretti PJ (2004). Let there be (NADH) light. Science 305: 50–52.
- Deutsch C, Taylor JS, Wilson DF (1982). Regulation of intracellular pH by human peripheral blood lymphocytes as measured by 19F NMR. Proc. Natl. Acad. Sci. U.S.A. 79 (24): 7944–7948.
- Gandhi GK, Cruz NF, Ball KK & Dienel GA (2009). Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. Journal of Neurochemistry 111(2): 522-536.
- Yellen G (2018). Fuelling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J Cell Biol 217 (7): 2235-2246.
- van Ginneken V, van den Thillart G, Muller H, van Deursen S, Onderwater M, Visee J, Hopmans V, van Vliet G, Nicolay K (1999). Phosphorylation state of red and white muscle in tilapia during graded hypoxia: an in vivo 31P-NMR study. Am J Physiol 277: R1501-R1512.
- Yu F-X, Dai R-P, Goh S-R, Zheng L & Luo Y (2009). Logic of a mammalian metabolic cycle: An oscillated NAD+/NADH redox signaling regulates coordinated histone expression and S-phase progression. Cell Cycle (Georgetown, Tex.) 8(5): 773–779.
- Norbury C & Nurse P (1992). Animal cell cycles and their control. Rev. Biochem. 6: 441-468.
- Diaz-Moralli S, Tarrado-Castellarnau M, Miranda A, Cascante M (2013). Targeting cell cycle regulation in cancer therapy. Ther. 138: 255–271.
- Plog BA & Nedergaard M (2018). The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annual Review of Pathology 13: 379-394.
- Ide K, Schmalbruch IK, Quistorff B, Horn A., & Secher NH (2000). Lactate, glucose and O2 uptake in human brain during recovery from maximal exercise. The Journal of Physiology 522 Pt 1(Pt 1): 159-164.
- Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, Deane R, … Nedergaard M (2016). Glymphatic clearance controls state-dependent changes in brain lactate concentration. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism 37(6): 2112-2124.
- van Ginneken V, Verheij E, de Vries E, van der Greef (2016). The discovery of two novel biomarkers in a high-fat diet C56bl6 Obese Model for Non-adipose tissue: a Comprehensive LCMS Study at Hind Limb, Heart, Carcass Muscle, Liver, Brain, Blood Plasma and Food Composition Following a lipidomics LCMS-Based Approach. Cellular & Molecular Medicine: Open access vol.2 No.2:13.
- Cook HW & McMaster CR (2002). Fatty acid desaturation and chain elongation in eukaryotes Chapter 7; 24 pp DE Vance & JE Vance [Eds] Biochemistry of Lipids, Lipoproteins and Membranes (4th Ed), Elsevier Science BV.
- Sun RC & Denko NC (2014). Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab 19: 285-292.
- Santos CR & Schulze A (2012). [Mini-review]: Lipid metabolism in cancer FEBS Journal 279: 2610-2623.
- Rӧhrig F & Schulze A (2016). The multifaceted roles of fatty acid synthesis in cancer. Nature Reviews Cancer 16: 732-749.
- Bulla LA & Cheng TC (2013). Invertebrate Models for Biomedical Research: Comparative Pathobiology, 167 pp.
- Parent A & Carpenter MB (1995). "Ch. 1". Carpenter's Human Neuroanatomy. Williams & Wilkins.
- Clark G (1969). World Prehistory: A New Outline; CUP Archive; 331 pages.
- Shultz, S., Nelson, E., & Dunbar, R. I. (2012). Hominin cognitive evolution: identifying patterns and processes in the fossil and archaeological record. pHilosopHical transactions of the Royal Society of London. Series B, Biological sciences, 367(1599), 2130–2140.
- Bar-Yosef O (2002). The Upper Paleolithic Revolution. Annual Review of Anthropology 31: 363-393.
- Nowell A (2010). Defining Behavioral Modernity in the Context of Neandertal and Anatomically Modern Human Populations. Annual Review of Anthropology 39: 437-452.
- Ambrose SH (2001). Paleolithic technology and human evolution. Science 291: 1748–1753.
- d'Errico F & Stringer CB (2011). Evolution, revolution or saltation scenario for the emergence of modern cultures? Trans. R. Soc. B 366: 1060-1069.
- McBrearty S & Brooks AS (2000). The revolution that wasn't: a new interpretation of the origin of modern human behavior. Hum. Evol. 39: 453–563.
- Spoor F, Wood B, Zonneveld F (1994). Implications of early hominid labyrinthine morphology for evolution of human bipedal locomotion. Nature 369 (6482): 645–648.
- Stringer CB (1994). Evolution of Early Humans. In: Steve Jones, Robert Martin, David Pilbeam. The Cambridge Encyclopedia of Human Evolution. Cambridge: Cambridge University Press; ISBN 978-0-521-32370-3; 242 pp.
- Craig S, Allen JS, Anton SC (2012). Biological Anthropology (2nd Edition); Chapter 1; Prentice Hall.
- Boyd R & Silk JB (2003). How Humans Evolved. New York, New York: Norton.
- Brues, A.M. & Snow, C.C. (1965). Physical Anthropology, Biennial Review of Anthropology 4: 1–39.
- Bramble DM & Lieberman DE (2004). Endurance running and the evolution of Homo. Nature 432 (7015): 345–352.
- Organ, C. (2012). Phylogenetic rate shifts in feeding time during the evolution of Homo. PNAS 108(35):14555-14559.
- Shultz S, Opie C, & Atkinson QD (2011). Stepwise evolution of stable sociality in primates. Nature, 479 (7372), 219–222.
- Povinelli DJ & Preuss TM (1995). Theory of mind: evolutionary history of a cognitive specialization. Trends Neurosci 18: 418-424.
- Mitteroecker P; Gunz P; Bernhard M; Schaefer K; Bookstein FL (2004). Comparison of cranial ontogenetic trajectories among great apes and humans. J. Hum. Evol. 46 (6): 679–97.
- Park MS, Nguyen AD, Arjan HE, Hoi-Sang U, Levy ML, Semendeferi K (2007). Evolution of the human brain: changing brain size and the fossil record. Neurosurgery 60 (3): 555–562.
- Bruner E (2007). Cranial shape and size variation in human evolution: structural and functional perspectives . Child's Nervous System 23(12): 1357-1365.
- Potts R (2012). Evolution and Environmental Change in Early Human Prehistory. Annu. Rev. Anthropol 41: 151–167.
- Leonard WR, Snodgrass JJ, Robertson ML (2007). Effects of Brain Evolution on Human Nutrition and Metabolism. Rev. Nutr. 27:311–327.
- Gibbons A (1998). Solving the Brain's Energy Crisis. Science 280: 1345–1347.
- Howell F & Bourlière F (2011). African Ecology and Human Evolution; Transaction Publishers; ISBN 978-0-202-36136-9; 398 pp.
- Leonard, W., Robertson, M., Aiello, L., & Wheeler, P. (1996). On Diet, Energy Metabolism, and Brain Size in Human Evolution. Current Anthropology, 37(1), 125-129.
- Müller AE & Thalmann U (2000). Origin and evolution of primate social organisation: a reconstruction. Biological Reviews of the Cambridge pHilosopHical Society, 75(3), 405–435.
- Ma, H., Marti-Gutierrez, N., Park, S.-W., Wu, J., Lee, Y., Suzuki, K., … Mitalipov, S. (2017). Correction of a pathogenic gene mutation in human embryos. Nature, 548(7668) : 413–419.
- van Ginneken V (2019). Multiple “Genetic Bottleneck Theory” of Humans and the House Mouse via Chilling Enzyme ∆12-Desaturase. Gastroenterology & Hepatology International Journal 4(2):000156; 22 pp.; ISSN: 2574-8009.
- van Ginneken V, Verheij E, van der Greef J (2019). Hypothesis on the human brain ‘Chilling enzyme’ ∆12 desaturase, the Toba volcano eruption and its glacial period, and the dispersal of Homo sapiens towards the Americas during the last glacial period J. Neurol Neurosci : 10.
- Rose WI & Chesner CA (1987). Dispersal of Ash in the Great Toba Eruption, 75 ka. Geology 15: 913-917.
- Ambrose SH (1998). Late Pleistocene human population bottlenecks, volcanic winter, and differentiation of modern humans. Journal of Human Evolution 34(6): 623-651.
- Rampino MR & Ambrose SH (2000). Volcanic winter in the Garden of Eden: The Toba super eruption and the late Pleistocene human population crash. In McCoy, FW; Heiken, G Volcanic Hazards and Disasters in Human Antiquity. Boulder, Colorado: Geological Society of America Special Paper, pp: 345.
- Wishart, D. S., Tzur, D., Knox, C., Eisner, R., Guo, A. C., Young, N., … Queren-gesser, L. (2007). HMDB: the Human Metabolome Database. Nucleic Acids Research, 35(Database): D521–D526.
- McGuire WJ (2007). The GGE Threat: Facing and Coping with Global Geophysical Events. In Bobrowsky, Peter T; Rickman, Hans. Comet/Asteroid Impacts and Human Society: an Interdisciplinary Approach. Springer, pp: 123-141.
- Rampino MR & Self S (1993). Climate-Volcanism Feedback and the Toba Eruption of ~74,000 Years ago. Quaternary Research 40(3): 269-280.
- Gibbons A (1993). Pleistocene Population Explosions. Science 262 (5130): 27-28.
- Robock A, Ammann CM, Oman L, Shindell D, Levis S, et al. (2009). Did the Toba volcanic eruption of ∼74k BP produce widespread glaciation? J Geophys Res 114: 9.
- Tattersall I (2017). Why was human evolution so rapid? in Human Paleontology and Prehistory, eds A. Marom and E. Hovers (New York, NY: Springer), 1–9.
- Cassirer, E. (1974). An Essay on Man: An Introduction to a pHilosophy of Human Culture. New Haven: Yale University Press, 1974:25-26.
- Bluff LA, Weir AS, Rutz C, Wimpenny JH, and Kacelnik A (2007). Tool-related cognition in New Caledonian crows. Cogn. Behav. Rev. 2: 1–25.
- Jaubert J, Verheyden S, Genty D., Soulier M, Cheng H, Blamart D et al. (2016). Early Neanderthal constructions deep in bruniquel cave in southwestern France. Nature 534: 111–114, 18291.
- Lewontin RC (1998). The evolution of cognition: questions we will never answer; in: An Invitation to Cognitive Science: Methods, Models, and Conceptual Issues, Vol. 4, eds D. Scarborough and S. Sternberg (Cambridge, MA: MIT Press), 107-132.
- Holloway RL, Broadfield DC, and Yuan MS (2004). Brain endocasts: the paleo-neurological evidence, in The Human Fossil Record, Vol. 3, eds J. H. Schwartz and I. Tattersall (New York, NY: Wiley-Liss).
- Finlay BL (2009). Brain evolution: developmental constraints and relative developmental growth; in: Encyclopedia of Neuroscience, Vol. 2, ed L. R. Squire (Oxford: Oxford University Press), 337–345.
- Semaw S, Renne P, Harris JWK, Feibel CS, Bernor RL, Fesseha N et al (1997).5-million-year- old stone tools from Gona, Ethiopia. Nature 385: 333–336.
- Kimbel WH & Lockwood CA (1999). Endocranial Capacity of Early Hominids. Science 283 (5398): 9.
- Durrleman, S., Pennec, X., Trouvé, A., Braga, J., Gerig, G., & Ayache, N. (2012). Toward a Comprehensive Framework for the Spatiotemporal Statistical Analysis of Longitudinal Shape Data. International Journal of Computer Vision, 103(1): 22–59.
- Herculano-Houzel S, Avelino-de-Souza K, Neves K, Porfírio J, Messeder D, Mattos Feijó L, Maldonado J, Manger PR (2014). The elephant brain in numbers. Frontiers in Neuroanatomy 8: 46.
- von Bartheld CS, Bahney J, Herculano-Houzel S (2016). The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. The Journal of Comparative Neurology 524 (18): 3865–3895.
- Schnack HG, van Haren NEM, Brouwer RM, Evans A, Durston S, Boomsma DI, Kahn RS, Hulshoff-Pol HE (2015). Changes in Thickness and Surface Area of the Human Cortex and Their Relationship with Intelligence. Cerebral Cortex 25 (6): 1608–1617.
- Rakic P (2009). Evolution of the neocortex: Perspective from developmental biology. Nature Reviews Neuroscience 10 (10): 724–735.
- Laurent G, Fournier J, Hemberger M, et al. (2016). Cortical Evolution: Introduction to the Reptilian Cortex. 2016 May 3. In: Buzsáki G, Christen Y, editors. Micro-, Meso- and Macro-Dynamics of the Brain.
- Lui JH, Hansen DV, Kriegstein AR (2011). Development and Evolution of the Human Neocortex. Cell 146 (1): 18–36.
- Miyagawa S, Lesure C, Nόbrega VA (2018). [Perspectives]: Cross-Modality Information Transfer: A Hypothesis about the Relationship among Prehistoric Cave Paintings Symbolic Thinking, and the Emergence of Language. Front Psychol. 2018; 9:115.
- Chomsky N (2016). What Kind of Creatures are we? New York, NY: Colombia University Press.
- Huijbregts, M. A. C. R. (2017). Phonemic clicks and the mapping asymmetry: how language emerged and speech developed. Neurosci. Biobehav. Rev. 81, 279–294.
- Thackeray F (2005). Eland, hunters and concepts of ‘sympathetic control’ expressed in southern African rock art. Archaecol. J. 15: 27–34.
- Plummer T (2004). Flaked stones and old bones: Biological and cultural evolution at the dawn of technology. Am. J. Phys. Anthropol. Suppl 39: 118–164.
- Crawford M (2002). Cerebral Evolution. Health 16: 29–34.
- Eastman CJ & Zimmerman MB (2018). The Iodine Deficiency Disorder. Endotext [Internet]: Up to date information on IDD can be obtained by visiting the website of the Iodine Global Network (IGN).
- Hohman G, Ortman S, Remer T, Fruth B (2019). Fishing for iodine: What Aquatic Foraging by Bonobos tells us about Human Evolution. BMC Zoology 4(5).
- Bundy WM (2007). Out of Chaos: Evolution from the Big Bang to Human Intellect, University Publishers; 509 pages.
- King MC & Wilson AC (1975). Evolution at two levels in humans and chimpanzees, Science 188: 107-116.
- Morange M (2011). The genetic distance between humans and chimpanzees: What did Mary-Claire king and Allan Wilson really say in 1975? Journal of Biosciences, 36(1): 23–26.
- Xu AG, He L, Li Z, Xu Y, Li M, Fu X et al. (2010). Intergenic and repeat transcription in human, chimpanzee and macaque brains measured by RNA-Seq. PLoS Comput. Biol. 6, e1000843–e1000843.
- Lu Y, Wang X, Yu H, Li J, Jiang Z, Chen B, Lu Y, Han C, Wang W, Ouyang Y, Huang L, Chen C, Tian W and Ling F (2018). Evolution and Comprehensive Analysis of DNase I Hypersensitive Sites in Brain-Related Genes Regulatory Regions of Primates. Genet. 10:152.
- Reilly, S. K., Yin, J., Ayoub, A. E., Emera, D., Leng, J., Cotney, J., et al. (2015). Evolutionary genomics. Evolutionary changes in promoter and enhancer activity during human corticogenesis. Science 347, 1155–1159.
- Doan, R. N., Bae, B. I., Cubelos, B., Chang, C., Hossain, A. A., Al-Saad, S., et al.(2016). Mutations in human accelerated regions disrupt cognition and social Cell 167, 341–354.e12.
- Brandler, W. M., Antaki, D., Gujral, M., Kleiber, M. L., Whitney, J., Maile, M.S., et al. (2018). Paternally inherited cis-regulatory structural variants are associated with autism. Science 360, 327–331.
- Balter, M. (2005). Evolution: Are Human Brains Still Evolving? Brain Genes Show Signs of Selection. Science, 309(5741), 1662–1663.
- Oldham MC & Geschwind DH (2005). Evolutionary Genetics: The human brain – adaptation at many levels. European Journal of Human Genetics, 13(5), 520–522.
- Kitano H (2002). Systems Biology: a brief overview. Science 295(5560): 1662-1664.
- Ridgway N & McLeod RS (editors) (2016). Book 6th Edition: Biochemistry of Lipids, Lipoproteins and Membranes; ISBN: 978-0-444-63438-2; Elsevier Science; 612 pp.
- Dennis EA. (2009). lipidomics joins the omics evolution. Proceedings of the National Academy of Sciences of the United States of America, 106(7), 2089–2090.
- van Ginneken V, Ham L, de Vries E, Verheij E, van der Greef J, van Eck M. (2016). Comparison of Hormones, Lipoproteins and Substrates in Blood Plasma in a C57bl6 Mouse Strain after Starvation and a High Fat Diet: A Metabolomics Approach. Anat Physiol 6:233; 5 pages.
- van Ginneken V, Verheij E, Hekman M, van der Greef J, Feskens E, Poelmann R (2011). The comparison of lipid profiling in Mouse brain and liver after starvation and a high-fat diet: a Medical Systems Biology approach. Chapter 3 page 151-186. In: Biology of Starvation in Humans and other organisms. Editor: Todd C. Merkin, ISBN: 978-1-61122-546-4, Nova Science Publishers, Inc.
- van Ginneken VJT, Booms R, Verheij E, de Vries E, van der Greef J (2016). The Relation between Non-adipose Muscle Fat and Hepatic Steatosis Studied with Localized 1H Magnetic Resonance Spectroscopy (1H MRS) and LCMS Anat Physiol 6: 245.
- van Ginneken V, de Vries E, Verheij E, van der Greef J (2016). Metabolomics in Hind Limb and Heart Muscle of a Mouse model after a High-Fat Diet. Anat Physiol 2016 6:3; ISSN: 2161-0940; 9pp.
- van Ginneken V, Verheij E, van der Greef J (2019). Impact of the ‘wrong’ fats on the composition of the heart muscle in an obese High Fat diet (HF-diet) induced Insulin Resistant C57bl6 mouse model following a lipidomics Systems Biology LCMS approach. EJPMR 6: 243-262.
- van Ginneken V (2017) Targeting Tumour Metabolism: A Biochemical Explanation Related to A Systems Biology lipidomics Based Approach. J Mol Biomark Diagn S2: 022.
- van Ginneken V, de Vries E, Verheij E, van der Greef J (2017). [Review]: Potential Biomarkers for “Fatty Liver” (Hepatic Steatosis) and Hepatocellular Carcinoma (HCC) and an explanation of their pathogenesis. Gastroenterol Liver Clin Med 1:1 001; 14 pp.
- van Ginneken V, Verheij E, Hekman M, van der Greef J (2017). Characterization of the lipid profile post mortem for Type-2 diabetes in human brain and plasma of the elderly with LCMS-techniques: a descriptive approach of diabetic encephalopathy. Integr Mol Med 4(2): 1-10.
- van Ginneken V, de Vries E, Verheij E, van der Greef J (2017). Type 3 Diabetes Reflects Disordered Lipid Metabolism in the Human Brain Related to Higher Degree of Unsaturated Fatty Acids Composition and is not Related to Body Mass Index. J Bioanal Biomed 9: 159-163.
- van Ginneken V, Verheij E, van der Greef J (2019). “Foetal Origins of Mental Diseases” Hypothesis Explained by a MUFAs towards PUFAs Obstruction via the Enzyme Stearoyl CoA Desaturase (SCD) in Post-Mortem Human Brain EC Physiology and Psychiatry 8.6 (2019).
- 1van Ginneken VJT, Verheij E, Hekman M, van der Greef J, Feskens EJM, Poelmann RE. (2013). The comparison of lipid profiling in mouse brain and liver after starvation and a high-fat diet: A medical systems biology approach Low and High-Fat Diets: Myths Vs. Reality. 105-140; Nova Science Publishers
- van Ginneken V, van Meerveld A, Verheij E, van der Greef J (2017). On the Futile Existence of DHA, None of EPA and the Predominant Role of the Triacylglycerols (TGs) in the Post Mortem Human Brain: An LCMS Study with Evolutionary Implications. J Bioanal Biomed 9: 152-158. doi:10.4172/1948-593X.1000170
- López, S., Van Dorp, L., & Hellenthal, G. (2015). Human Dispersal Out of Africa: A Lasting Debate. Evolutionary Bioinformatics, 11s2, EBO.S33489. doi:10.4137/EBO.S33489.
- Wolpoff, M., Spuhler, J., Smith, F., Radovcic, J., Pope, G., Frayer, D., … Clark, G. (1988). Modern human origins. Science, 241(4867), 772–774. doi:10.1126/science.3136545
- Wolpoff, M. H., Wu, X. Z., & Alan, G., G. Thorne (1984). Modern Homo sapiens Origins: A General Theory of Hominid Evolution Involving the Fossil Evidence from east Asia. The Origins of Modern Humans, Liss, New York, 411–83.
- Wolpoff, M. H., Hawks, J., & Caspari, R. (2000). Multiregional, not multiple origins. American Journal of Physical Anthropology, 112(1), 129–136.
- Thorne AG & Wolpoff MH (2003). The Multiregional Evolution of Humans. Scientific American 13(2):46-53.
- Templeton A (2002). Out of Africa again and again. Nature 416(6876): 45–51. doi:10.1038/416045a
- Yuan, Dejian; Lei, Xiaoyun; Gui, Yuanyuan; Zhu, Zuobin; Wang, Dapeng; Yu, Jun; Huang, Shi (2018). Modern human origins: multiregional evolution of autosomes and East Asia origin of Y and mtDNA. bioRxiv: 101410.
- Fagundes, N. J., Ray, N., Beaumont, M., Neuenschwander, S., Salzano, F. M., Bonatto, S. L., & Excoffier, L. (2007). Statistical evaluation of alternative models of human evolution. Proceedings of the National Academy of Sciences of the United States of America, 104(45): 17614–17619.
- Relethford JH (2001). Genetics and the Search for Modern Human Origins (Wiley, New York, 2001).
- Cann RL; Stoneking M. & Wilson, AC (1987). Mitochondrial DNA and human evolution. Nature 325:31-36.
- Vigilant, L., Stoneking, M., Harpending, H., Hawkes, K. & Wilson, A. C. (1991). African populations and the evolution of human mitochondrial DNA. Science 253:1503-1507.
- Stoneking M & Soodyall H (1996). Human evolution and the mitochondrial genome. Curr. Opin. Genet. Dev. 6: 731-736.
- Mellars, P. (2006). Why did modern human populations disperse from Africa ca. 60,000 years ago? A new model. Proceedings of the National Academy of Sciences, 103(25): 9381–9386. doi:10.1073/pnas.0510792103
- Innis SM (2003). Perinatal biochemistry and physiology of LCPUFA. J Pediatr 143: S1-S8.
- Innis SM (2007). Dietary (n-3) fatty acids and brain development. J. Nutr. 137: 855-859.
- Van Ginneken V & Löwik C (2018). Extension of the “Foetal Origin Hypothesis of Barker” towards the “Foetal Origin Hypothesis of Mental Diseases”, J Psychology and Mental Health Care. 7 pages.