ES Journal of Dental Sciences
ISSN: 2768-0126
Can Tobacco consumption explain the association between SEP and chronic periodontitis in adults living in a deprived area of the UK? A secondary analysis of the ELOHI study data
Research Article
- Mahwish Anjum1,2, David G Gillam1* and Wagner Marcenes3
- 1Institute of Dentistry, Bart’s and the London School of Medicine and Dentistry, Queen Mary University of London
- 2Present address University of Greenwich, London, UK
- 3King’s College London Dental Institute, Division of Population and Patient Health, King’s College, UK
- *Corresponding author: David G Gillam, Oral Bioengineering, Institute of Dentistry, Barts and the London School of Medicine and Dentistry QMUL, Whitechapel, London E12AD, United Kingdom
- Received: Feb 09, 2020; Accepted: Feb 29, 2020; Published: Mar 11, 2020
- Copyright: 2020 © David G Gillam. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: To assess whether there are social inequalities on periodontitis at the population level in a deprived area of the United Kingdom (UK) and further elucidate the relationship between socio-economic position, plaque accumulation, tobacco smoking, and chronic periodontitis in a representative sample of adults living in a multicultural and socially deprived area.
Methods: The present sub study used the cross-sectional data previously collected in the East London Oral Health Inequality (ELOHI) study, conducted in the outer northeast boroughs of London, UK. A stratified two-staged sampling was performed which consisted of a sample of 2149 adults (16-65 years of age). Participants underwent an oral examination and answered a supervised questionnaire in their own homes. Data regarding information on the explanatory variables: socio-economic position (SEP), oral health behaviour and indicators were obtained from the ELOHI study. The main outcome variable for the present sub study was chronic periodontitis (presence of at least one site of a tooth with a pocket depth (PD) ≥4mm). Statistical analysis included conceptual hierarchical modelling and mediation analysis. The level of statistical significance was set at 0.05.
Results: The prevalence of periodontitis in this area of East London was very high, 80.5% among males and 82% among females although these differences were not statistically significant. Hierarchical conceptual modelling analysis demonstrated that those in the manual/routine occupations category were 2.21 (95% CI 1.64-2.989) more likely to have chronic periodontitis than those in the professional category. The difference between those in the intermediate and the professional and managerial occupations category were not statistically significant after adjusting for demographic and behavioural variables. The results of mediation analysis, using the four steps proposed by Baron and Kenny demonstrated that the association between socio-economic position and periodontitis was partially mediated through smoking.
Conclusion: A social gradient in periodontal diseases in part mediated by tobacco consumption may exist even in areas where there are highly socially deprived communities was identified. SEP as measured by NS-SEC was found to be associated with the chronic periodontitis experience (PD ≥ 4mm) with individuals placed higher in the NS-SEC ranking demonstrating a lesser risk of disease as compared to a lower NS-SEC ranking.
Introduction
Severe chronic periodontitis (SCP) was listed on the top ten most prevalent cause of health loss around the globe among all 313 diseases assessed in the Global Burden of Diseases, Injuries, and Risk Factors 2015 (GBD) Study [1]. The global age-standardised prevalence of SCP was 7.4% affecting 538 million (95% C.I.: 465-626) people in 2015. The number of incident cases of SCP in 2015 was six (95% C.I.: 5.0-6.6) million people worldwide. Therefore, it is a significant global health challenge [1-2].
Periodontitis is an inflammatory disease of the supporting structures of teeth (e.g., connective tissues and alveolar bone) which is initially induced by bacteria and the subsequent development of periodontitis is related to the response of the periodontal tissues to pathogens [3]. Furthermore, the development of specific bacteria types has been consistently identified from over 500-700 species in the microbial flora in the oral cavity such as Porphyromonasgingivalis, Tannerella forsythia and Aggregatibacter actinomycetemcomitans have been implicated as aetiological agents for periodontitis. The likelihood for the development of periodontitis in an individual, harbouring one of these periodontal pathogens may not, necessarily be sufficientt o be considered as a risk factor [4]. There is also a low strength of evidence which suggests that more frequent professional plaque removal (PMPR) is associated with improved plaque and bleeding outcomes and possibly less annual attachment loss. Needleman et al. [5] in a systematic review, based on a moderate strength of evidence from the literature reported there is no additional benefit to plaque and gingival bleeding outcomes from PMPR over that achieved by repeated and thorough OHI. The quantitative and qualitative composition of the resident microflora, which is dictated by behavioural risk factors, may, however play an important role in the development of periodontitis. For example, inadequate oral hygiene leads to increased dental plaque accumulates beyond levels compatible with oral health, leading to shifts in the balance of the predominant bacteria away from levels associated with health [6]. Risk factors such as systemic conditions (Type II diabetes; glycaemic control; cardiovascular disease, rheumatoid arthritis), local factors (crowns, bridges, defective restorative margins) and behaviour factors (alcohol intake, smoking, oral hygiene) can also have an impact on the oral microbial flora which in turn may initiate inflammatory changes that can be observed clinically [7,8]. However, these factors based on biological/behavioural models of do not fully explain the variation of the presence of gingival bleeding [7,9].
There is however evidence that tobacco smoking is associated with poor oral hygiene and is a relevant behavior risk factor for the development of periodontitis [10-12]. Further more, there is strong evidence that SEP affects tobacco smoking [13-14], plaque accumulation and dental attendance [7,15]. Regardless of the socio-economic indicator used, persons who are socio-economically disadvantaged consistently have poorer periodontal health behavior [9,16-17].
Finally, periodontitis is socially patternedand influenced by psychological factors, both Sheiham & Nicolau and Borrell et al.[18-19] reviewed the evidence on the association between socio-economic position and periodontitis and concluded that regardless of the socioeconomic indicator used, those who are socio-economically disadvantaged consistently have poorer periodontal health than those who are socio-economically advantaged. These authors also highlighted the need to investigate the role of socio-economic position (SEP) on the causal pathway. Periodontal diseases models should be updated to the socioeconomic- epidemiology era, where a hierarchical approach from the molecular to the societal level is proposed. Zini et al. [20] suggested a potential explanatory pathway for the relationship between SEP and SCP and proposed SEP as a distal determinant, leading to tobacco smoking and higher levels of plaque, and finally to SCP.
Aim
This paper aimed to assess whether there are social inequalities on periodontitis at the population level in a deprived area of the United Kingdom (UK) and further elucidate the relationship between socio-economic position, plaque accumulation, tobacco smoking, and chronic periodontitis in a representative sample of adults living in a multicultural and socially deprived area.
Methods
This article analysed data from the East London Oral Health Inequality (ELOHI) Study, which included adults 16 to 65 years old living in Waltham Forest, Redbridge and Barking and Dagenham in 2009-10. The Outer North East London Research Ethics Committee approved the study protocol (08/H0701/93). Participants provided written informed consent agreeing their voluntary participation.
The ELOHI study adopted a cross-sectional study design. A multi-stage stratified random sampling approach was used to select a representative sample of the ethnically diverse general non-institutionalised population in East London. The sampling frame was a list of all addresses stratified by the number of wards in Barking and Dagenham (n=17), Redbridge (n=21) and Waltham Forest (n=20). Fifty-five addresses were randomly selected from each ward to yield 3,193 addresses. Residents were then contacted by post and invited to participate in the study. Non-respondents were visited to ascertain the household was empty and age of residents. We excluded 457 commercial premises or vacant addresses and 208 ineligible households with no residing adults age 16 to 65 years. The final sampling frame included 2,528 valid addresses and 1,437 (56%) households agreed to participate in the study. The household response rate in the Barking and Dagenham, Redbridge and Waltham Forest wards was 61%, 52.2% and 61.2% respectively, which represented a total response rate of 57%. Non-respondents were replaced by inviting residents in the same postcode area. As a result, the mean Index of Multiple Deprivation (IMD) scores in the sample and for the population in 2007 were 33.46 and 34.45 respectively. A maximum of two adults per household were invited to participate in the study, and all agreed yielding a sample of 2,343 adults that successfully completed an oral examination and reported their age, gender and ethnicity.
Participants underwent an oral examination and answered a supervised questionnaire in their own homes. Purpose trained and calibrated dentists performed the oral examinations using the UK Adult Dental Health Survey 1998 protocol [21] and standardised equipment (e.g. Daray light lamps, mirror and periodontal probes). Participants’ teeth were not brushed or professionally cleaned prior to examination, but debris and moisture were removed from individual sites with cotton wool rolls or cotton buds if visibility was obscured and probes were used for cleaning debris from the tooth surfaces to enable visual examination. The examination included a full mouth assessment for presence of both plaque and periodontal pockets. Pocket depths were measured at two sites (mesial and distal) on each tooth (bucally (facially) for the upper teeth and lingually for the lower teeth) and the worst score for each tooth was coded. Disposable periodontal probes with coloured-coded bands were used. Duplicate examinations were performed among participants to assess intra examiner agreement within a two-week interval. Examiners’ assessments were individually compared with the reference examiner assessment under field circumstances. The Kappa value (n=133 subjects) for dental status at tooth level was 0.83, which indicated excellent agreement.
Following the clinical examination participants answered a supervised self-completed questionnaire. The questionnaire included questions on socio-demographic factors (age, gender, socio-economic position, education) and oral health behaviour and status. Individuals’ SEP was measured by education and the National Statistics Socio-Economic Classification (NS-SEC) [22]. Education was indicated by the highest degree or qualification (no qualifications, secondary school, A levels, technical qualifications, first university degree or higher degree). NSSEC groups were derived using the self-coded method based on current or last main job or occupation, employment status, size of organisation and supervisory status. Five operational categories were derived: (1) managerial and professional, (2) intermediate, (3) small employers and own account workers, (4) lower supervisory and technical, and (5) semi-routine and routine occupations. For complete coverage of the population, full-time students, individuals who had never worked or were in long-term unemployment and those not classified for other reasons were added as not classified [22]. Behavioural variables included tobacco smoking.
Statistical analysis
The data were weighted to adjust for the unequal probability of selection and non-response and to produce a representative sample (with respect to age, gender and ethnicity) based on the United Kingdom (UK) Census in 2001 [23]. Weighting the data did not increase the size of the sample (weighted data=2,266 adults) and accounted for the weighting of data and the complex survey design (stratification and clustering) to produce corrected standard errors and confidence intervals. This data analysis further excluded 117 participants due to missing data on periodontal health status. Therefore, the sample size for this sub-study included 2149 adults. Post-hoc calculation also demonstrated that the minimum sample size to provided 80% statistical power to identify an odds ratio of 1.5 and/or a risk ratio of 1.2 was estimated to be 822 [24]. The calculation assumed 50% of the unexposed population and 60% of the exposed population to have the outcome of interest, α equal to 0.05, and β equal to 0.20.
Data manipulation was minimal. Age was categorised into ten-years brackets. Education was re-categorised into four groups, namely no qualification, secondary school, A levels and higher education. Socio-economic classification was further categorised into managerial and professional, intermediate, routine and manual occupations and not classified. Periodontitis was defined by the presence of at least one site with periodontal pocket depth ≥ 4mm as reported in the UK Adult Dental Health Survey 1998 protocol [21]
Simple logistic regression analyses were performed to assess the unadjusted association between each of the independent variables (socio-economic position, sex, age, tobacco consumption, last visit to the dentist and presence of plaque) and chronic periodontitis. In accordance with the lax criterion [25], explanatory variables that were not statistically significantly related to the outcome at the level of 0.20 were excluded at this stage. Following this, conceptual hierarchical modelling was performed [26]. The remaining variables were sequentially included as follows: (1) socio-economic position and age, (2) plus tobacco consumption (3) plus last visit to the dentist(4) plus presence of plaque. Odds Ratios (OR) were reported and the 95% confidence interval was considered. Mediation analysis included the four steps proposed by Baron and Kenny [27]. Attenuation of the OR was calculated using the formula (ORU-ORA)÷(ORU-1) (25), where ORU represents the odds ratio before including tobacco consumption and ORA reflects the odds ratio after including tobacco consumption in the model.
Results
2266 weighted sample size for adults, aged 16 to 65 years old, were included in the present study, hundred and seventeen adults were excluded as they were either edentate or they lacked evidence on their periodontal status, which in turn resulted in missing information in the outcome variable. As a result of this exclusion, the final sample size for this sub study was 2149 adults.
The demographic and socioeconomic characteristics of the sample are shown in Table 1. The mean age of the participants was 40.3 years old of whom 48.5% (1043 of 2149) were males. A greater proportion of the sample was from the White British (67.2%) ethnic group. Approximately30% of the total sample population belonged to routine or manual occupations while 40% belonged to the managerial and professional group.Presence of visible dental plaque in this sample was high (85%) and only 52% attended a dental setting in the last year. (Table 1).
Table 1: Frequency distribution of demographic and socio-economic variables in a sample of 2149 (16 to 65 years old) adults in East London.
|
Variables |
Frequency |
% |
|
Gender |
|
|
|
Male |
1043 |
48.5 |
|
Female |
1106 |
51.5 |
|
NS-SEC |
|
|
|
Managerial and professional occupations |
862 |
40.1 |
|
Intermediate occupations |
179 |
8.3 |
|
Small employers and own account workers |
168 |
7.8 |
|
Lower supervisory and technical occupations |
203 |
9.5 |
|
Semi-routine and routine occupations |
272 |
12.6 |
|
Never worked and long term unemployment |
249 |
11.6 |
|
Full time students |
114 |
5.3 |
|
Missing Information |
100 |
4.7 |
|
Tobacco Use |
|
|
|
No |
1275 |
59.3 |
|
Yes |
468 |
21.8 |
|
Missing |
|
|
|
Last dental Visit |
|
|
|
≤1 year |
881 |
40.9 |
|
≥1 year |
655 |
30.5 |
|
Missing |
|
|
|
Visible Plaque |
|
|
|
No |
217 |
10.1 |
|
Yes |
1530 |
71.2 |
|
Periodontitis outcome |
|
|
|
PP<4mm |
403 |
18.7 |
|
PP≥4mm |
1746 |
81.3 |
The prevalence of periodontitis in East London was very high, 80.5% among males and 82% among females. Gender differences were not statistically significant. There was a statistically significant gradual increase in the prevalence of periodontitis with the increasing age of the participants. Unadjusted results also showed a statistically significant association between periodontitis and socio-economic position,tobacco consumption, last visit to the dentist and presence of dental plaque (Table 2).
Table 2: Unadjustedodds ratios and 95% confidence intervals of demographic, socio-economic and periodontal health behaviour variables on chronic periodontitis in a sample of 2149 (16 to 65 years old) adults living in East London.
|
Variables |
Chronic Periodontitis |
||
|
N (mean or % with disease outcome) |
ORa (95% CI) |
p-value |
|
|
Age |
? |
1.04 (1.03-1.05) |
<0.001 |
|
Gender |
|
|
|
|
Male |
840 (80.5) |
1 [reference] |
|
|
Female |
907 (82.0) |
1.10 (0.88-1.37) |
0.37 |
|
NS-SEC |
|
|
|
|
Managerial and professional occupations |
670 (77.7) |
1 [reference] |
|
|
Intermediate occupations |
151 (84.4) |
1.55 (1.006-2.39) |
0.047 |
|
Routine and manual occupations |
561 (87.2) |
1.95 (1.47- 2.59) |
<0.001 |
|
Never worked and long term unemployment |
211 (84.4) |
1.56 (1.07- 2.28) |
0.020 |
|
Full time students |
80 (70.2) |
0.68 (0.44- 1.05) |
0.087 |
|
Missing |
|
|
|
|
Tobacco Use |
|
|
|
|
No |
1275 (79.7) |
1 [reference] |
0.006 |
|
Yes |
468 (85.9) |
1.53 (1.17- 2.01) |
0.002 |
|
Missing |
|
|
|
|
Last Dental Visit |
|
|
|
|
≤1 year |
881 (79.0) |
1 [reference] |
|
|
≥1 year |
655 (85.5) |
1.56 (1.22- 2.00) |
<0.001 |
|
Missing |
|
|
|
|
Visible Plaque |
|
|
|
|
No |
217 (67.8) |
1 [reference] |
|
|
Yes |
1530 (83.6) |
2.43 (1.86- 3.17) |
<0.001 |
Hierarchical conceptual modelling analysis (Table 3) demonstrated that those in the manual/routine occupations category were 2.21(95% CI 1.64-2.989) more likely to have chronic periodontitis than those in the professional category, however the difference between those in the intermediate and the professional and managerial occupations category were not statistically significant after adjusting for demographic and behavioural variables (Table 3). Tobacco smoking and last visit to the dentist were also significantly related to chronic periodontitis. The social gradient was attenuated after adjustment for age, gender, and tobacco smoking and last visit to the dentist (Table 3).
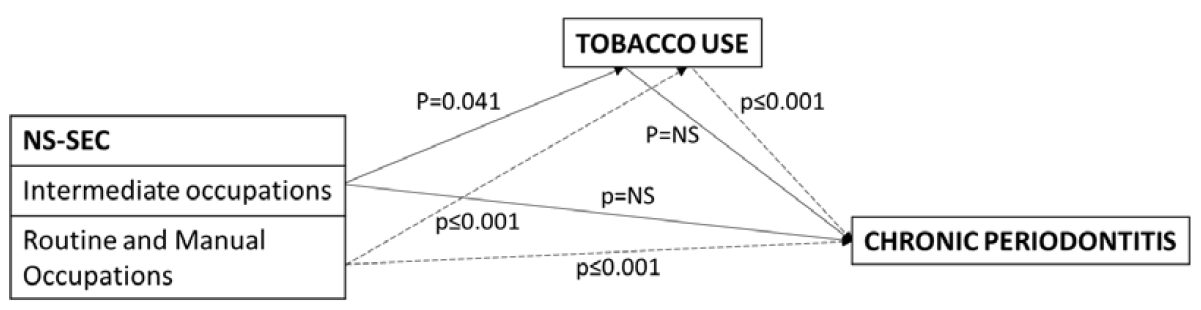
The results of mediation analysis, using the four steps as proposed by Baron and Kenny [27] (Figure 1) confirmed that the association between socio-economic position and periodontitis was partially mediated through smoking. Socio-economic position was significantly associated with smoking and periodontitis, smoking was associated with periodontitis and the likelihood that those in routine and manual and intermediate occupations would have periodontitis compared to those in managerial and professional occupations was attenuated after adjusting for smoking (Table 3).
Table 3: Results of the conceptual hierarchical modelling of demographic, socio-economic and periodontal health behaviour variables on chronic periodontitis in a sample of 2149 (16 to 65 years old) adults living in East London.
|
Variables |
Model 1a |
P value |
Model 2a |
P value |
Model 3a |
P value |
|
ORb (95% CI) |
ORb (95% CI) |
ORb (95% CI) |
||||
|
SEP |
|
|
|
|
|
|
|
Managerial and professional occupations |
1.00 [Reference] |
|
1.00 [Reference] |
|
1.00 [Reference] |
|
|
Intermediate occupations |
1.63 [1.05-2.53] |
0.031 |
1.59 [1.02-2.47] |
0.041 |
1.52 [0.97-2.37] |
0.066 |
|
Routine and Manual Occupations |
2.33 [1.74-3.11] |
<0.001 |
2.18 [1.62-2.92] |
<0.001 |
1.99 [1.47-2.68] |
<0.001 |
|
Never worked and long-term unemployment |
2.17 [1.47-3.22] |
<0.001 |
2.19 [1.48-3.26] |
<0.001 |
1.96 [1.31-2.92] |
0.001 |
|
Full time students |
1.41 [0.88-2.27] |
0.153 |
1.33 [0.83-2.15] |
0.329 |
1.19 [0.73-1.93] |
0.489 |
|
Tobacco Use |
|
|
|
|
|
|
|
No |
|
|
1.00 [Reference] |
|
1.00 [Reference] |
|
|
Yes |
|
|
1.51 [1.14-1.99] |
0.004 |
1.43 [1.07-1.90] |
0.015 |
|
Last Dental Visit |
|
|
|
|
|
|
|
≤1 year |
|
|
|
|
1.00 [Reference] |
|
|
≥1 year |
|
|
|
|
1.09 [0.771.53] |
0.639 |
|
Visible Plaque |
|
|
|
|
|
|
|
No |
|
|
|
|
1.00 [Reference] |
|
|
Yes |
|
|
|
|
2.58 [1.94-3.42] |
<0.001 |
|
a Model 1 was SEP adjusted for age; Model 2 was SEP adjusted for age, plus tobacco use; Model 3 was SEP adjusted for age plus, tobacco use, last dental visit and visible plaque;
|
||||||
Discussion
Some limitations of the present study need to be addressed before discussing and comparing the outcomes with those in the published literature. For example, the secondary analysis of the ELOHI study data as reported in this paper was based on the 1999 classification of periodontal diseases which has recently be superseded by the 2018 World workshop [28]. Previous studies on the inequalities in health have been based on the previous 1999 classification of periodontal disease although it should be recognised that the terminology to define the extent and severity of the disease process has changed and may therefore be redundant for future studies [16,21]. It should also be noted that the present study analysed crosssectional data which limits the ability to establish causal relationships between variables. Furthermore, since the study sample only included 2149 out of 2266 (95%) adults who participated in the ELOHI study this may raise some concerns about its representativeness and the effect of the missing data on the results. However, no significant differences in socio-demographic composition between our study sample and the full sample of ELOHI participants was observed, which would indicate that missing data are unlikely to have impacted the results and that they can therefore be generalized to the study population.
Socio-economic inequalities in periodontal diseases exist at global, national and community level both in adults and children [7,13-18]. The findings of the present study in East London showed that socio-economic inequalities in periodontal diseases exist even among those living in adeprived area despite there being less variation in the index of multiple deprivation scores. East London is characterised by a population that includes residents from diverse ethnic backgrounds and high levels of social deprivation. These findings are important because they provide strong support to the social gradient concept.
Those individuals consuming tobacco were more likely to have the experience of chronic periodontitis (expressed as having at least one tooth with PD ≥4mm) [21] when compared to non-consumers of tobacco. This association established (at 95% C.I.) an OR of 1.53, for those consuming tobacco as opposed to non-consumers, which was even after adjusting for confounding variables was statistically significant and in agreement with previous studies in the literature [29-31]. However, no evidence of an association between the dose dependency, the effect of frequency and duration of exposure to tobacco was demonstrated in the present study.
Other oral health behaviour and indicators, for example visits to the dentist and percentage of teeth with visible plaque were also statistically significantly associated with the disease experience as defined in the present sub study
There was a significant association between the SEP and chronic periodontitis when assessing the SEP of the study population in accordance with the individual based socioeconomic indicator that is, NS-SEC. An association between SEP and chronic periodontitis has been demonstrated in the published literature [32]. In the present sub study with regard to NS-SEC association, the routine and manual occupation’s participants were 1.93 times more likely to experience periodontal disease when compared to those in the managerial occupations category This social gradient which was statistically significant appeared to confirm the previous outcomes reported in the literature [13,18]. There may however be a possibility of over representation of the managerial and professional category at the top of the NS-SEC hierarchy in the present study as these individuals were more likely to be motivated and willing to take participate in such oral health studies [9].
In addition, the results of the hierarchical conceptual modelling analysis demonstrated that tobacco consumption mediated the association between SEP and periodontitis. Public health initiatives therefore must consider prevent and manage chronic periodontitis through integrated disease prevention strategies based on a common risk factor approach. The Common Risk Factor Approach (CRFA) has been highly influential in integrating oral health into general health improvement strategies [33]. It is also important to stress that the behavioural preventive approach alone will have minimal impact in tackling oral health inequalities and indeed may widen inequalities across the population [13]. The results from the present studywould appear to support the social determinants agenda and theconceptual framework adopted to analyse the datain the study accounted for the relevant influence of confounding risk factors.
Conclusion
A social gradient in periodontal diseases in part mediated by tobacco consumption may exist even in areas where there are highly socially deprived communities was identified. SEP as measured by NS-SEC was found to be associated with the chronic periodontitis experience (PD ≥ 4mm) with individuals placed higher in the NS-SEC ranking demonstrating a lesser risk of disease as compared to a lower NS-SEC ranking.
Another important conclusion was the independent association of tobacco consumption with disease experience (PD ≥ 4mm). Other oral health behaviour and indicators, for example visits to the dentist and percentage of teeth with visible plaque were also statistically significantly associated with the disease experience as defined in the present sub study.
Acknowledgement
This study was conducted by the Institute of Dentistry, Barts and The London School of Medicine and Dentistry, Queen Mary University of London (QMUL), in collaboration with Redbridge, Waltham Forest and Barking and Dagenham Primary Care Trusts (PCTs) to inform planning and commissioning of dental care services. We are grateful for the support of the participants involved in this study. We also thank all individuals who helped to organise and execute the ELOHI study.
Authors Contributions
All authors contributed to data analysis plan, selection of key covariates, wrote and reviewed the manuscripts. WM conceived the study and oversaw the implementation and conducting of the fieldwork, analysed the data and provided overall guidance.
References
- Kassebaum NJ, Smith AGC, Bernabé E, Fleming TD, Reynolds AE, Vos T, Murray CJL, Marcenes W; GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and DisabilityAdjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J Dent Res 2017; 96(4) 380–387.
- Tonetti M,Jepsen S, Jin L, Otomo-Corgel J. Impact of the global burden of periodontal diseases on health,nutrition and wellbeing of mankind: A call for global action. J Clin Periodontol. 2017;44:456–462.
- Kornman KS. Mapping the pathogenesis of periodontitis: a new look. J Periodontol,2008, 79, 1560-8.
- Ezzo PJ; Cutler CW. Microorganisms as risk indicators for periodontal disease. Periodontol 2000,2003; 32, 24-35.
- Needleman IA, Nibali L;Di Iorio A. Professional mechanical plaque removal for prevention of periodontal diseases in adults – systematic review update. J Clin Periodontol2015; 42 (Suppl. 16): S12–S35. doi: 10.1111/jcpe.12341.
- Nazir MA. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int J Health Sci (Qassim). 2017 Apr-Jun; 11(2): 72–80.
- Koga R, Herkrath APCQ, Vettore MV, Herkrath FJ, Rebelo Vieira JM, Pereira JV, Rebelo MAB, Queiroz AC. The role of socioeconomic status and psychosocial factors on gingivitis in socially disadvantaged adolescents. J Periodontol. 2020 Feb;91(2):223-231. doi: 10.1002/JPER.19-0129. Epub 2019 Sep 3.
- Murakami S, Mealey BL, Mariotti A, Chapple ILC. Dental plaque-induced gingival conditions. J Periodontol. 2018 Jun;89 Suppl 1:S17-S27. doi: 10.1002/JPER.17-0095. Review.
- Hakeem FF, Sabbah W. Is there socioeconomic inequality in periodontal disease among adults with optimal behaviours. Acta Odontol Scand. 2019 Jul;77(5):400-407. doi: 10.1080/00016357.2019.1582795. Epub 2019 Mar 28.
- Albandar JM, Streckfus CF, Adesanya MR; Winn DM. Cigar, pipe, and cigarette smoking as risk factors for periodontal disease and tooth loss. J Periodontol, 2000; 71, 1874-81.
- Hujoel P, Berström J, Del Aguila MA; De Rouen TA. A hidden periodontitis epidemic during the 20th Century? Community Dentistry and Oral Epidemiology. 2003;31:1–6. doi: 10.1034/j.1600-0528.2003.00061.x.
- Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol, 2000; 71, 743-51.
- Watt R; Sheiham A. Inequalities in oral health: a review of the evidence and recommendations for action. Br Dent J, 1999; 187, 6-12.
- Sabbah W, Tsakos G, Sheham A; Watt RG. The role of health-related behaviors in the socioeconomic disparities in oral health. Soc Sci Med, 2009; 68, 298-303.
- Listl S. Inequalities in dental attendance throughout the life-course. J Dent Res, 91, 2012; 91s-97s.
- Borrell LN, Burt BA, Warren RC,; Neighbors HW. The role of individual and neighborhood social factors on periodontitis: the third National Health and Nutrition Examination Survey. J Periodontol, 77, 2006; 444-53.
- Buchwald S, Kocher T, Biffar R, Harb A, Holtfreter B; Meisel P. Tooth loss and periodontitis by socio-economic status and inflammation in a longitudinal population-based study. J Clin Periodontol, 2013; 40, 203-11.
- Sheiham A; Nicolau B. Evaluation of social and psychological factors in periodontal disease. Periodontol 2000, 2005; 39, 118-31.
- Borrell LN, Burt BA, Gillespie BW, Lynch J; Neighbors H. Periodontitis in the United States: beyond black and white. J Public Health Dent, 2002; 62, 92-101.
- Zini A, Sgan-Cohen HD; Marcenes W. Socio-economic position, smoking, and plaque: a pathway to severe chronic periodontitis. J Clin Periodontol, 38, 2011; 229-35.
- Kelly M, Steele J, Nuttall N M, Bradnock G, Morris J, Nunn J, Pine C, Pitts N B, Treasure E, White D, Walker A, Cooper I. Adult Dental Health Survey. Oral Health in the United Kingdom. The Stationary Office, London., 2000.
- Office For National Statistics. The National Statistics Socio-Economic Classification: user manual. 2005; Basingstoke: Palgrave Macmillan, UK.
- Office For National Statistics. Census 2001 [Online].
- Fleiss JL, Tytun A; Ury HK. A Simple Approximation for Calculating Sample Sizes for Comparing Independent Proportions. Biometrics, 36, 1980; 343-346.
- Altman DG. Practical statistics for medical research. 1991; London: Chapman and Hall.
- Victoria C, Huttly S, Fuchs S; Olinto M. The role of conceptual frameworks in epidemiological analysis: a hierarchical approach. International Journal of Epidemiology. 1997;26(1):224–227. pmid:9126524.
- Baron RM; Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical considerations. Journal of Personality and Social Psychology, 1986; 51, 1173-1182.
- Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, Tonetti MS. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Periodontol. 2018 Jun;89 Suppl1:S1-S8. doi: 10.1002/JPER.18-0157.
- Bergström J, Preber H. Tobacco Use as a Risk Factor. J Periodontol. 1994 May;65 Suppl 5S:545-550.
- Sham AS, Cheung LK, Jin LJ, Corbet EF.The effects of tobacco use on oral health.Hong Kong Med J. 2003 Aug;9(4):271-7.
- Honkala S, Honkala E, Newton T, Rimpelä A. Toothbrushing and smoking among adolescents--aggregation of health damaging behaviours. J Clin Periodontol. 2011 May; 38(5):442-8.
- Susin C, Haas AN, Valle PM, Oppermann RV, Albandar JM. Prevalence and risk indicators for chronic periodontitis in adolescents and young adults in south Brazil.J Clin Periodontol. 2011 Apr;38(4):326-33.
- Sheiham A, Watt RG. The common risk factor approach: a rational basis for promoting oral health. Community Dent Oral Epidemiol. 2000 Dec;28(6):399-406.