ES Journal of Case Reports
Serious Infection of Chest Wall during Radiotherapy in Breast Cancer Patient with Large Seroma: A Case Report and Review of the Literature
Case Presentation
- Guanqiao Li1#, Jia Yao1#, Baizhen Cai2, Qiang Fu2, Fei Kong2, and Shiping Yang2*
- 1Department of Breast surgery, Hainan General Hospital (Hainan Affiliated Hospital of Medical University), China
- 2Department of Radiation Oncology, Hainan General Hospital (Hainan Affiliated Hospital of Medical University), China
- *Corresponding author: Shiping Yang, Department of Radiation Oncology, Hainan General Hospital, NO.19, Xiu hua Road, Xiuying district, Haikou, Hainan, PR China.
- Received: Feb 12, 2021; Accepted: Mar 01, 2021; Published: Mar 03, 2021
- Copyright: 2021 © Shiping Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: A seroma is the most common complication after modified radical mastectomy for breast cancer. We could not find any publications about chest wall infections in patients with large seromas during radiotherapy in Pubmed.
Case presentation: We report a 59-year woman with breast cancer. The seroma gradually enlarged during the course of chemotherapy. Severe infections occurred on chest wall during radiotherapy. The patient’s abscesses were cut and discharged. Pathogen culture and drug sensitive test had was performed. Pseudomonas aeruginosa found in pus culture. They were then given antibiotics. The patient underwent three rounds of painful debridement and negative pressure treatment. Finally debridement and flap plasty was performed on the patient. After a series of operations, the patient no longer had seroma and recovered well. She remains free of locoregional recurrence or distant metastases after 37 months of postoperative follow-up. It is hoped that, following this paper, more attention will be paid to patients with large seromas in the pre-chemotherapy or pre-radiotherapy assessment of breast cancer.
Conclusion: Serious infection of would occur on the breast cancer patient with large seroma, especially when they underwent chemotherapy. Multidisciplinary treatment is deed when the seroma is large. We would suggest that it is necessary to drain the fluid area first before chemotherapy or radiotherapy when the seroma is large.
Keywords: Breast cancer; Large seroma; Radiotherapy; Chemotherapy; Case report
Abbreviations ER: Estrogen Receptor; PR: Progesterone Receptor; Her-2: Human Epidermal Growth Factor Receptor-2; CT: Computed Tomography; AC: Adjuvant Chemotherapy
Introduction
Background
Modified radical mastectomy is common treatment for breast cancer [1]. A seroma is the most common complication of breast cancer after modified radical mastectomy. Usually, subcutaneous effusion is called a seroma. It is defined as a serous fluid collection that develops under the skin flaps or in the axillary dead space after axillary dissection [2]. The occurrence of seromas after radical mastectomy was 15%~60%, the highest rate was up to 85% [3-6]. It is considered a side effect of surgery rather than a complication. However, none of the patients had symptoms [7]. Most seromas were small and could be absorbed after surgery in time. However, in our case, the seroma does not only absorb, it becomes very large after chemotherapy, which was infected seriously during radiotherapy. We could not find any publications about chest wall infections in patients with large seromas during radiotherapy in Pubmed. This is the first time data related to chest wall infections in patients with large seromas during radiotherapy will be published. It is hoped that, following this paper, more attention will be paid to patients with large seromas in the pre-radiotherapy assessment of breast cancer.
Case Presentation
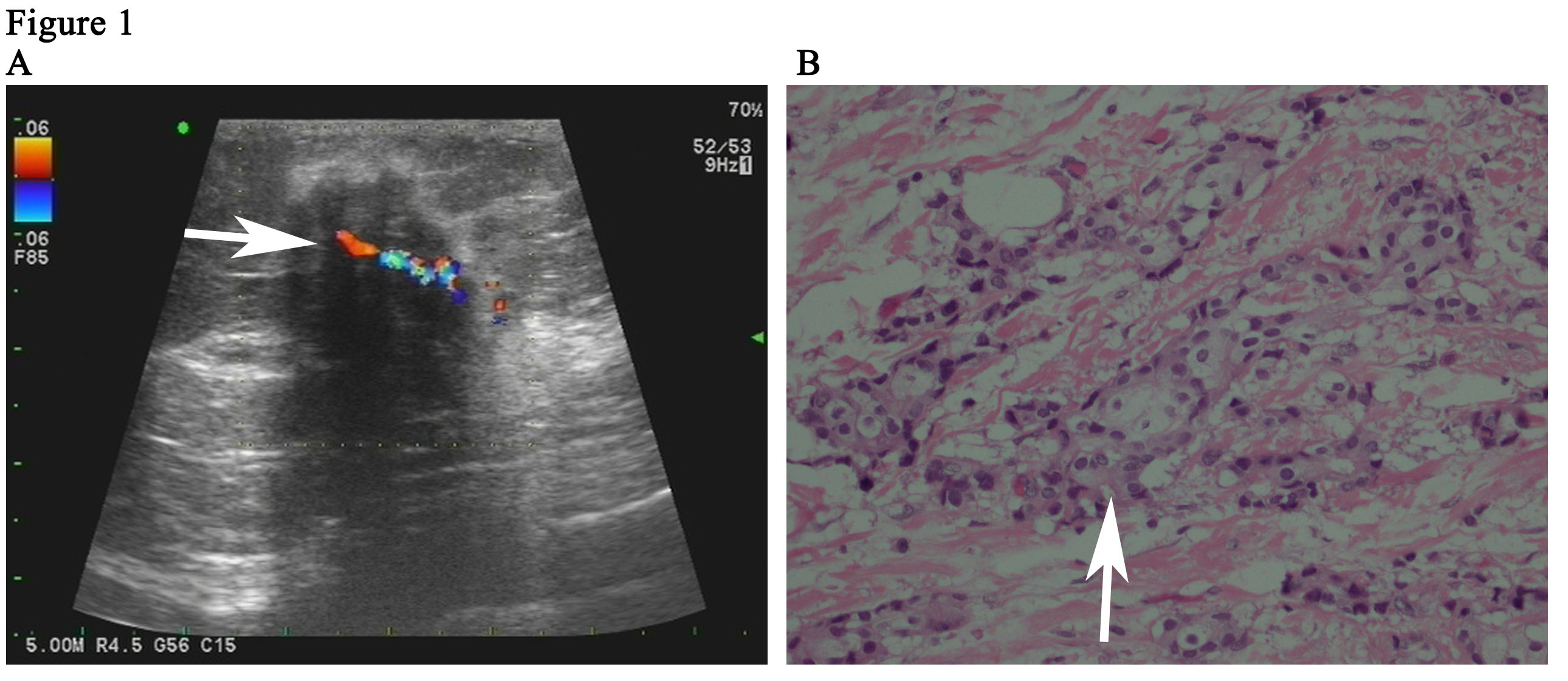
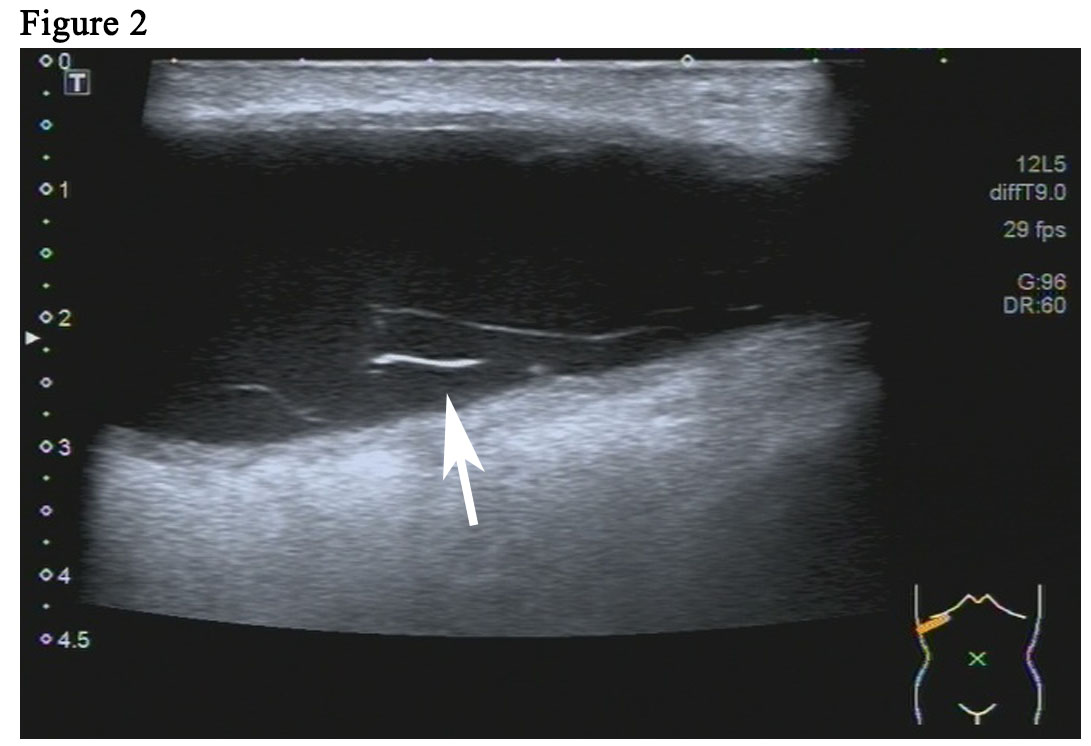
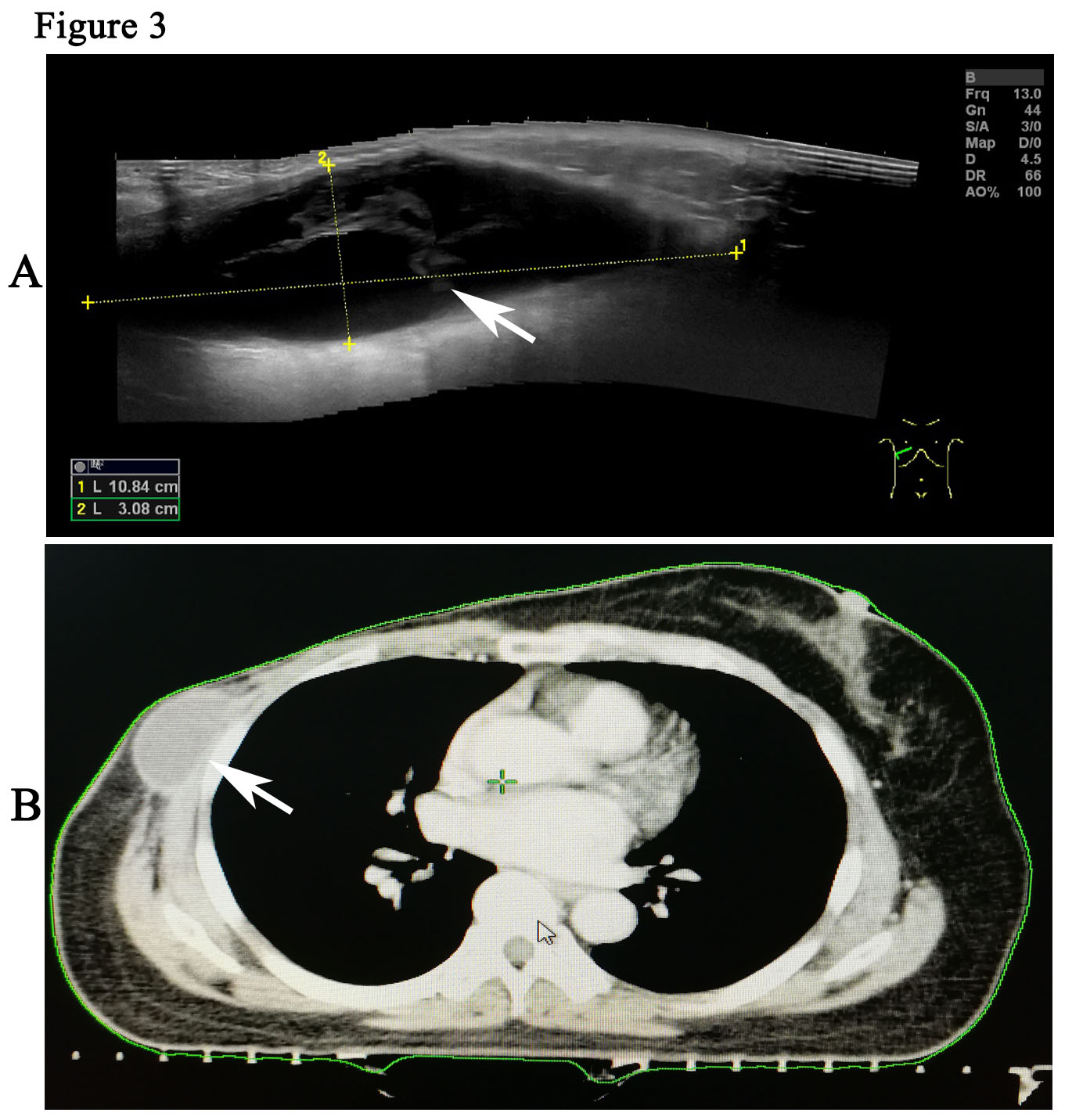
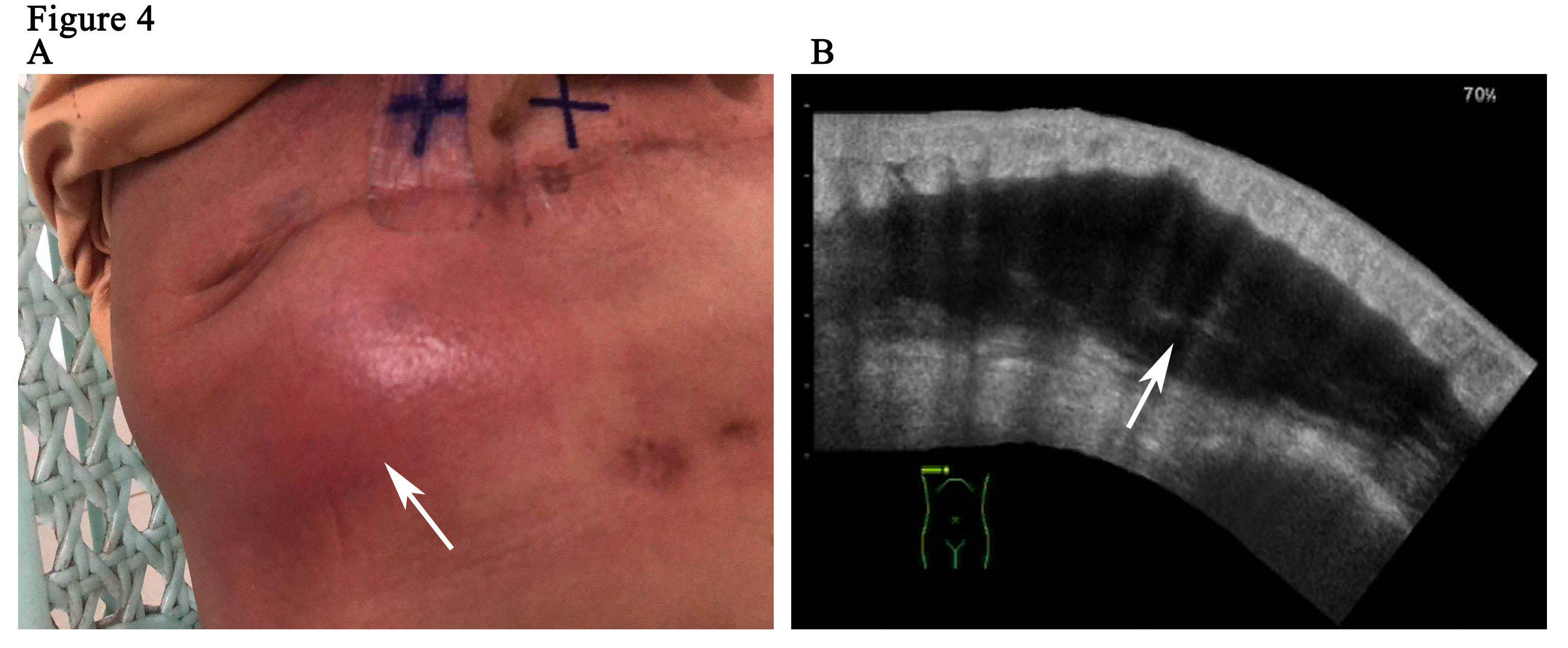
The patient is a 59-year woman. She had a mass for one month in the left upper outer quadrant region of the right breast. The patient had no past medical history or family history of breast cancer. Physical examination showed a mass of 3cm × 3cm sized, which was mildly tender and firm. The surface skin of mass was not ruptured. Lymph nodes of axillary and supraclavicular were not palpable. Ultrasound examination (Figure 1A) revealed a welldefined hypoechoic mass without calcification or evidence of invasion, which was 3.0cm × 2.6cm in size for the breast. The markers of erum tumor and other routine blood tests were normal. The bdominal and axillary lymph node ultrasound, chest X-ray and bone scan were normal. The patient was performed an excisional biopsy as an outpatient. Histopathology of a fast frozen section results showed an invasive ductal carcinoma. Following the diagnosis, she underwent a modified radical operation. The pathologist also confirmed there was an invasive ductal carcinoma (Figure 1B). 7 of the 22 dissected axillary lymph nodes demonstrated evidence of disease and the surgical margins were negative. The estrogen receptor (ER) was positive at 100%, progesterone receptor (PR) and human epidermal growth factor receptor-2 (Her-2) was negative. The tumor was pathologically staged as pT2N2M0 according with the eighth edition of the AJCC cancer [8]. An ultrasound of the patient’s breast was performed before she undertook the first cycle of chemotherapy. This ultrasound revealed a seroma of right chest wall measuring 7cm × 2.4cm in size (Figure 2). Then, the patient was performed adjuvant chemotherapy (AC) (doxorubicin + cyclophosphamide for 4 cycles, following by docetaxel cycled for 4 cycles). The patient then had another ultrasound of her breast after she had finished the last cycle of chemotherapy. The results of this ultrasound showed that the seroma of right chest wall now measured 10.8cm × 3.1 cm in size (Figure 3A). CT (computed tomography) scan also suggested a large seroma of the chest wall (Figure 3B). The seroma of the chest wall was enlarged compared with its size before starting chemotherpy. Once the round of chemotherapy was complete the patient then received adjuvant radiation therapy. The patient was treated with intensity modulate radiation therapy (IMRT). The planned dose was 50Gy (2.0 Gy/fraction x 25 fractions) to the affected areas (chest wall and regional lymph node). However, the patient had to stop radiotherapy when the accumulated dose was 38Gy, because she developed a soft tissue infection of chest wall. The right chest wall of secroma became red, swollen and painful. The region of infection was the same area where the seroma was (Figure 4A). Abscess had formed under the chest wall. Ultrasound revealed dense fine-dotted echoes in seroma (Figure 4B). The patient experienced a recurrent fever which resulted in her highest temperature being 39.8 ℃. A routine blood test revealed that leukocytes (14.9*10E9/L) and neutrophils (12.4*10E9/L) had increased significantly. C reactive protein (56.11mg/L) showed similar increases. The patient was then admitted to the Skin Repair Department. She was diagnosed with the right chest wall of secroma infection and abscess formation. The patient’s abscess was cut and discharged. Pathogen culture and drug sensitive test had was perform. Pseudomonas aeruginosa found in pus culture. Piperacillin sodium sulbactam, sodium cefmetazole sodium and ofloxacin were high sensitivity in drug sensitive test. She was then given antibiotics. The patient underwent three rounds of painful debridement and negative pressure treatment. Finally debridement and flap plasty was performed on the patient who then remained hospitalized for 2 weeks and after which went home for recuperation. Following discharge, the patient no longer had seroma and recovered well. She remains free of locoregional recurrence or distant metastases after 37 months of postoperative follow-up.
Discussion
Although a seroma is not threatening for life, it could lead to significant serious compilations (e.g. predisposes to sepsis, flap necrosis, wound dehiscence, prolonged recovery period) [9,10]. Fluid collection is ideally managed by repeated needle aspiration to seal the skin flaps against the chest wall. Several factors (e.g. age, use of pressure garment, duration of wound drainage, postoperative arm activity, use of electrocautery and chemotherapy) have been investigated as the cause of seroma formation [11- 16]. A little subcutaneous effusion can be absorbed by themselves. If the adhesion between the flap and the chest wall is damaged, a pseudomembrane is formed. Then the effusion will be wrapped up. So the stubborn subcutaneous effusion cannot be effectively absorbed. This patient should belong to this kind of stubborn subcutaneous effusion, and the range of effusion increased after chemotherapy. The insufficient nutrition and increased range of shoulder movement led to the increase of the separation space between the flap and the chest wall, which resulted in the increase of liquid accumulation.
In this case, there was no basic disease, chemotherapy went smoothly, but the seroma gradually enlarged during the course of chemotherapy. Severe infections occurred on chest wall during radiotherapy because of the large size of the seroma (10.8 cm × 3.1 cm). When a large seroma occurs, we recommended that the effusion is treated with repeated needle aspiration before radiotherapy. It is not known if radiotherapy can be used to treat large seromas because there is no published literature in this area. We then tried to analyze the reasons for this. We came to the conclusions that possibly due to the large size of the seroma it is not easy to absorb in a short time. The complicated seroma has caused by surgical manipulation or distortion of the barrier effect of skin. No puncture culture was performed before irradiation, so it was not completely ruled out that bacteria were latent then, but the patient had no symptoms and signs of infection before irradiation. Seroma is rich in nutrition which is a natural medium for bacterial growth. This would support its rapid growth. After chemotherapy, the patient’s immunity is low. Furthermore, radiotherapy may lead to a decrease in the resistance of local areas to bacteria. At last, this opportunistic pathogen caused serious infections in the immunocompromised.
In this case, the main issue with the treatment that the patient finally received three debridement and flap plasty for recovery. Multidisciplinary treatment is a new trend. The patient had the opportunity to ask for a surgery consultation before radiotherapy or during chemotherapy. If the patient could be treated with extraction or drainage of effusion, perhaps the probability of infection will be greatly reduced, so as not to cause chest wall infection and interrupt radiotherapy.
Serious infection of would occur on the breast cancer patient with large seroma, especially when they underwent chemotherapy. Multidisciplinary treatment is deed when the seroma is large. We would suggest that it is necessary to drain the fluid area first before chemotherapy or radiotherapy when the seroma is large.
Consent for Publication
Written informed consent was obtained from the patient’s daughter for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgements
We are very grateful to Dean Moody who helped us in improving the English writing.
Funding Sources
This work was supported by Natural Science Foundation of Hainan Province,China (819QN345).
Author Contributions
Data acquisition: YS, YJ and FQ. Data analysis and interpretation: LG, KF and YJ. Radiological analysis of ultrasound and CT images: WZ. Manuscript preparation: LG and YS.
References
- Hashemi E, Kaviani A, Najafi M, Ebrahimi M, Hooshmand H, Montazeri A. Seroma formation after surgery for breast cancer. World J Surg Oncol. 2004; 2: 44.
- Pogson CJ, Adwani A, Ebbs SR. Seroma following breast cancer surgery. Eur J Surg Oncol. 2003; 29: 711-717.
- Chand N, Aertssen AMG, Royle GT. Axillary “Exclusion”-A Successful Technique for Reducing Seroma Formation after Mastectomy and Axillary Dissection. Advances in Breast Cancer Research. 2013; 2: 1-6.
- Gonzalez EA, Saltzstein EC, Riedner CS, Nelson BK. Seroma formation following breast cancer surgery. Breast J. 2003; 9: 385-388.
- Kuroi K, Shimozuma K, Taguchi T. Pathophysiology of seroma in breast cancer. Breast Cancer. 2005; 12: 288-293.
- Zieliński J, Jaworski R, Irga N, Kruszewski JW, Jaskiewicz J. Analysis of selected factors influencing seroma formation in breast cancer patients undergoing mastectomy. Arch Med Sci. 2013; 9: 86-92.
- Gunn J, Gibson T, Li Z, Diehl N, Bagaria S, McLaughlin S. Symptomatic Axillary Seroma after Sentinel Lymph Node Biopsy: Incidence and Treatment. Ann Surg Oncol. 2016; 23: 3347-3353.
- Giuliano AE, Edge SB, Hortobagyi GN. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann Surg Oncol. 2018; 25: 1783-1785.
- Budd DC, Cochran RC, Sturtz DL, Fouty WJ. Surgical morbidity after mastectomy operations. Am J Surg. 1978; 135: 218-220.
- Aitkin DR, Minton JP. Complications associated with mastectomy. Surg Clin North Am. 1983; 63: 1331-1352.
- Barwell J, Campbell L, Watkins RM, Teasdale C. How long should suction drains stay in after breast surgery with axillary dissection? Ann R Coll Surg Engl. 1997; 79: 435-437.
- Dawson I, Stam L, Heslinga JM, Kalsbeck HL. Effect of shoulder immobilization on wound seroma and shoulder dysfunction following modified radical mastectomy: a randomized prospective clinical trial. Br J Surg. 1989; 76: 311-312.
- Petrek JA, Peters MM, Nori S, Knaner C, Kinne DW, Rogatco A. Axillary lymphadenectomy: a prospective, randomized trial of thirteen factors influencing drainage, including early or delayed arm mobilization. Arch Surg. 1990; 125: 378-382.
- Gonzalez EA, Saltzstein EC, Riedner CS, Nelson BK. Seroma formation following breast cancer surgery. Breast J. 2003; 9: 385-388.
- Porter KA, O’Connor S, Rimm E, Lopez M. Electrocautery as a actor in seroma formation following mastectomy. Am J Surg. 1998; 176: 8-11.
- O’ Hea BJ, Ho MN, Petrek JA. External compression dressing versus standard dressing after axillary lymphadenectomy. Am J Surg. 1999; 177: 450-453.